
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding.

In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bond and is sometimes called an unshared pair or non-bonding pair. Lone pairs are found in the outermost electron shell of atoms. They can be identified by using a Lewis structure. Electron pairs are therefore considered lone pairs if two electrons are paired but are not used in chemical bonding. Thus, the number of electrons in lone pairs plus the number of electrons in bonds equals the number of valence electrons around an atom.

In chemistry, pi bonds are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals has an electron density of zero at a shared nodal plane that passes through the two bonded nuclei. This plane also is a nodal plane for the molecular orbital of the pi bond. Pi bonds can form in double and triple bonds but do not form in single bonds in most cases.
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds when a molecule is formed. In contrast, molecular orbital theory has orbitals that cover the whole molecule.

In chemistry, π backbonding, also called π backdonation, is when electrons move from an atomic orbital on one atom to an appropriate symmetry antibonding orbital on a π-acceptor ligand. It is especially common in the organometallic chemistry of transition metals with multi-atomic ligands such as carbon monoxide, ethylene or the nitrosonium cation. Electrons from the metal are used to bond to the ligand, in the process relieving the metal of excess negative charge. Compounds where π backbonding occurs include Ni(CO)4 and Zeise's salt. IUPAC offers the following definition for backbonding:
A description of the bonding of π-conjugated ligands to a transition metal which involves a synergic process with donation of electrons from the filled π-orbital or lone electron pair orbital of the ligand into an empty orbital of the metal (donor–acceptor bond), together with release (back donation) of electrons from an nd orbital of the metal (which is of π-symmetry with respect to the metal–ligand axis) into the empty π*-antibonding orbital of the ligand.
In chemistry, bond order is a formal measure of the multiplicity of a covalent bond between two atoms. As introduced by Linus Pauling, bond order is defined as the difference between the numbers of electron pairs in bonding and antibonding molecular orbitals.

In organometallic chemistry, the isolobal principle is a strategy used to relate the structure of organic and inorganic molecular fragments in order to predict bonding properties of organometallic compounds. Roald Hoffmann described molecular fragments as isolobal "if the number, symmetry properties, approximate energy and shape of the frontier orbitals and the number of electrons in them are similar – not identical, but similar." One can predict the bonding and reactivity of a lesser-known species from that of a better-known species if the two molecular fragments have similar frontier orbitals, the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). Isolobal compounds are analogues to isoelectronic compounds that share the same number of valence electrons and structure. A graphic representation of isolobal structures, with the isolobal pairs connected through a double-headed arrow with half an orbital below, is found in Figure 1.
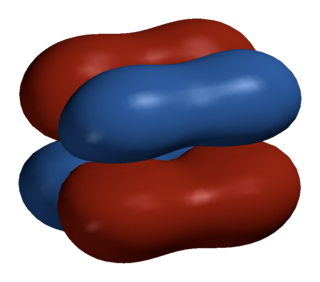
In chemistry, delta bonds are covalent chemical bonds, where four lobes of one involved atomic orbital overlap four lobes of the other involved atomic orbital. This overlap leads to the formation of a bonding molecular orbital with two nodal planes which contain the internuclear axis and go through both atoms.
A three-center two-electron (3c–2e) bond is an electron-deficient chemical bond where three atoms share two electrons. The combination of three atomic orbitals form three molecular orbitals: one bonding, one non-bonding, and one anti-bonding. The two electrons go into the bonding orbital, resulting in a net bonding effect and constituting a chemical bond among all three atoms. In many common bonds of this type, the bonding orbital is shifted towards two of the three atoms instead of being spread equally among all three. Example molecules with 3c–2e bonds are the trihydrogen cation and diborane. In these two structures, the three atoms in each 3c-2e bond form an angular geometry, leading to a bent bond.

A quintuple bond in chemistry is an unusual type of chemical bond, first reported in 2005 for a dichromium compound. Single bonds, double bonds, and triple bonds are commonplace in chemistry. Quadruple bonds are rarer and are currently known only among the transition metals, especially for Cr, Mo, W, and Re, e.g. [Mo2Cl8]4− and [Re2Cl8]2−. In a quintuple bond, ten electrons participate in bonding between the two metal centers, allocated as σ2π4δ4.

Chromium(II) acetate hydrate, also known as chromous acetate, is the coordination compound with the formula Cr2(CH3CO2)4(H2O)2. This formula is commonly abbreviated Cr2(OAc)4(H2O)2. This red-coloured compound features a quadruple bond. The preparation of chromous acetate once was a standard test of the synthetic skills of students due to its sensitivity to air and the dramatic colour changes that accompany its oxidation. It exists as the dihydrate and the anhydrous forms.

Diatomic carbon (systematically named dicarbon and 1λ2,2λ2-ethene), is a green, gaseous inorganic chemical with the chemical formula C=C (also written [C2] or C2). It is kinetically unstable at ambient temperature and pressure, being removed through autopolymerisation. It occurs in carbon vapor, for example in electric arcs; in comets, stellar atmospheres, and the interstellar medium; and in blue hydrocarbon flames. Diatomic carbon is the second simplest of the allotropes of carbon (after atomic carbon), and is an intermediate participator in the genesis of fullerenes.

A sextuple bond is a type of covalent bond involving 12 bonding electrons and in which the bond order is 6. The only known molecules with true sextuple bonds are the diatomic dimolybdenum (Mo2) and ditungsten (W2), which exist in the gaseous phase and have boiling points of 4,639 °C (8,382 °F) and 5,930 °C (10,710 °F) respectively.

Potassium octachlorodimolybdate is an inorganic compound with the chemical formula K4[Mo2Cl8]. It is known as a red-coloured, microcrystalline solid. The anion is of historic interest as one of the earliest illustrations of a quadruple bonding. The salt is usually obtained as the pink-coloured dihydrate.

Molybdenum(II) acetate is a coordination compound with the formula Mo2(O2CCH3)4. It is a yellow, diamagnetic, air-stable solid that is slightly soluble in organic solvents. Molybdenum(II) acetate is an iconic example of a compound with a metal-metal quadruple bond.
Transition metal carbyne complexes are organometallic compounds with a triple bond between carbon and the transition metal. This triple bond consists of a σ-bond and two π-bonds. The HOMO of the carbyne ligand interacts with the LUMO of the metal to create the σ-bond. The two π-bonds are formed when the two HOMO orbitals of the metal back-donate to the LUMO of the carbyne. They are also called metal alkylidynes—the carbon is a carbyne ligand. Such compounds are useful in organic synthesis of alkynes and nitriles. They have been the focus on much fundamental research.

Potassium octachlorodirhenate(III) is an inorganic compound with the formula K2Re2Cl8. This dark blue salt is well known as an early example of a compound featuring quadruple bond between its metal centers. Although the compound has no practical value, its characterization was significant in opening a new field of research into complexes with quadruple bonds.
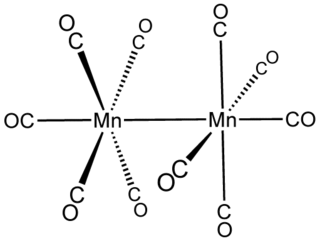
In inorganic chemistry, metal–metal bonds describe attractive interactions between metal centers. The simplest examples are found in bimetallic complexes. Metal–metal bonds can be "supported", i.e. be accompanied by one or more bridging ligands, or "unsupported". They can also vary according to bond order. The topic of metal–metal bonding is usually discussed within the framework of coordination chemistry, but the topic is related to extended metallic bonding, which describes interactions between metals in extended solids such as bulk metals and metal subhalides.
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except -2, although the oxidation states +7, +4, and +3 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. The tetrathioperrhenate anion [ReS4]− is possible.
Principal interacting orbital (PIO), based on quantum chemical calculations, provides chemists with visualization of a set of semi-localized dominant interacting orbitals. The method offers additional perspective to molecular orbitals (MO) obtained from quantum chemical calculations, which often provide extensively delocalized orbitals that are hard to interpret and relate with chemists' intuition on electronic structures and orbital interactions. Several other efforts have been made to help visualize semi-localized dominant interacting orbitals that represents well chemists' intuition, while maintaining the mathematical rigorosity. Notable examples include the natural atomic orbitals (NAO), natural bond orbitals (NBO), charge decomposition analysis (CDA), and adaptive natural density partitioning (AdNDP). PIO analysis uniquely provides semi-localized MOs that are chemically accurate and easy to interpret.

![The octachlorodirhenate(III) anion, [Re2Cl8] , which features a quadruple Re-Re bond Octachlorodirhenate(III)-3D-balls.png](http://upload.wikimedia.org/wikipedia/commons/thumb/2/2e/Octachlorodirhenate%28III%29-3D-balls.png/150px-Octachlorodirhenate%28III%29-3D-balls.png)














