Related Research Articles

A microRNA is a small single-stranded non-coding RNA molecule found in plants, animals and some viruses, that functions in RNA silencing and post-transcriptional regulation of gene expression. miRNAs function via base-pairing with complementary sequences within mRNA molecules. As a result, these mRNA molecules are silenced, by one or more of the following processes: (1) cleavage of the mRNA strand into two pieces, (2) destabilization of the mRNA through shortening of its poly(A) tail, and (3) less efficient translation of the mRNA into proteins by ribosomes.

Gene expression is the process by which information from a gene is used in the synthesis of a functional gene product that enables it to produce end products, protein or non-coding RNA, and ultimately affect a phenotype, as the final effect. These products are often proteins, but in non-protein-coding genes such as transfer RNA (tRNA) and small nuclear RNA (snRNA), the product is a functional non-coding RNA. Gene expression is summarized in the central dogma of molecular biology first formulated by Francis Crick in 1958, further developed in his 1970 article, and expanded by the subsequent discoveries of reverse transcription and RNA replication.
In molecular biology and genetics, transcriptional regulation is the means by which a cell regulates the conversion of DNA to RNA (transcription), thereby orchestrating gene activity. A single gene can be regulated in a range of ways, from altering the number of copies of RNA that are transcribed, to the temporal control of when the gene is transcribed. This control allows the cell or organism to respond to a variety of intra- and extracellular signals and thus mount a response. Some examples of this include producing the mRNA that encode enzymes to adapt to a change in a food source, producing the gene products involved in cell cycle specific activities, and producing the gene products responsible for cellular differentiation in multicellular eukaryotes, as studied in evolutionary developmental biology.

Regulation of gene expression, or gene regulation, includes a wide range of mechanisms that are used by cells to increase or decrease the production of specific gene products. Sophisticated programs of gene expression are widely observed in biology, for example to trigger developmental pathways, respond to environmental stimuli, or adapt to new food sources. Virtually any step of gene expression can be modulated, from transcriptional initiation, to RNA processing, and to the post-translational modification of a protein. Often, one gene regulator controls another, and so on, in a gene regulatory network.

In cell biology, a paraspeckle is an irregularly shaped compartment of the cell, approximately 0.2-1 μm in size, found in the nucleus' interchromatin space. First documented in HeLa cells, where there are generally 10-30 per nucleus, Paraspeckles are now known to also exist in all human primary cells, transformed cell lines and tissue sections. Their name is derived from their distribution in the nucleus; the "para" is short for parallel and the "speckle" refers to the splicing speckles to which they are always in close proximity. Their function is still not fully understood, but they are thought to regulate gene expression by sequestrating proteins or mRNAs with inverted repeats in their 3′ UTRs.
The Let-7 microRNA precursor was identified from a study of developmental timing in C. elegans, and was later shown to be part of a much larger class of non-coding RNAs termed microRNAs. miR-98 microRNA precursor from human is a let-7 family member. Let-7 miRNAs have now been predicted or experimentally confirmed in a wide range of species (MIPF0000002). miRNAs are initially transcribed in long transcripts called primary miRNAs (pri-miRNAs), which are processed in the nucleus by Drosha and Pasha to hairpin structures of about 70 nucleotide. These precursors (pre-miRNAs) are exported to the cytoplasm by exportin5, where they are subsequently processed by the enzyme Dicer to a ~22 nucleotide mature miRNA. The involvement of Dicer in miRNA processing demonstrates a relationship with the phenomenon of RNA interference.

The miR-9 microRNA, is a short non-coding RNA gene involved in gene regulation. The mature ~21nt miRNAs are processed from hairpin precursor sequences by the Dicer enzyme. The dominant mature miRNA sequence is processed from the 5' arm of the mir-9 precursor, and from the 3' arm of the mir-79 precursor. The mature products are thought to have regulatory roles through complementarity to mRNA. In vertebrates, miR-9 is highly expressed in the brain, and is suggested to regulate neuronal differentiation. A number of specific targets of miR-9 have been proposed, including the transcription factor REST and its partner CoREST.

The miR-129 microRNA precursor is a small non-coding RNA molecule that regulates gene expression. This microRNA was first experimentally characterised in mouse and homologues have since been discovered in several other species, such as humans, rats and zebrafish. The mature sequence is excised by the Dicer enzyme from the 5' arm of the hairpin. It was elucidated by Calin et al. that miR-129-1 is located in a fragile site region of the human genome near a specific site, FRA7H in chromosome 7q32, which is a site commonly deleted in many cancers. miR-129-2 is located in 11p11.2.
Small activating RNAs (saRNAs) are small double-stranded RNAs (dsRNAs) that target gene promoters to induce transcriptional gene activation in a process known as RNA activation (RNAa).

RNA interference (RNAi) is a biological process in which RNA molecules are involved in sequence-specific suppression of gene expression by double-stranded RNA, through translational or transcriptional repression. Historically, RNAi was known by other names, including co-suppression, post-transcriptional gene silencing (PTGS), and quelling. The detailed study of each of these seemingly different processes elucidated that the identity of these phenomena were all actually RNAi. Andrew Fire and Craig C. Mello shared the 2006 Nobel Prize in Physiology or Medicine for their work on RNA interference in the nematode worm Caenorhabditis elegans, which they published in 1998. Since the discovery of RNAi and its regulatory potentials, it has become evident that RNAi has immense potential in suppression of desired genes. RNAi is now known as precise, efficient, stable and better than antisense therapy for gene suppression. Antisense RNA produced intracellularly by an expression vector may be developed and find utility as novel therapeutic agents.

mir-127 microRNA is a short non-coding RNA molecule with interesting overlapping gene structure. miR-127 functions to regulate the expression levels of genes involved in lung development, placental formation and apoptosis. Aberrant expression of miR-127 has been linked to different cancers.
In molecular biology, the miR-200 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by binding and cleaving mRNAs or inhibiting translation. The miR-200 family contains miR-200a, miR-200b, miR-200c, miR-141, and miR-429. There is growing evidence to suggest that miR-200 microRNAs are involved in cancer metastasis.
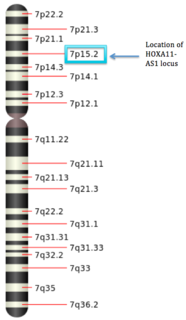
HOXA11-AS lncRNA is a long non-coding RNA from the antisense strand in the homeobox A. The HOX gene contains four clusters. The sense strand of the HOXA gene codes for proteins. Alternative names for HOXA11-AS lncRNA are: HOXA-AS5, HOXA11S, HOXA11-AS1, HOXA11AS, or NCRNA00076. This gene is 3,885 nucleotides long and resides at chromosome 7 (7p15.2) and is transcribed from an independent gene promoter. Being a lncRNA, it is longer than 200 nucleotides in length, in contrast to regular non-coding RNAs.

Cancer epigenetics is the study of epigenetic modifications to the DNA of cancer cells that do not involve a change in the nucleotide sequence, but instead involve a change in the way the genetic code is expressed. Epigenetic mechanisms are necessary to maintain normal sequences of tissue specific gene expression and are crucial for normal development. They may be just as important, or even more important, than genetic mutations in a cell's transformation to cancer. The disturbance of epigenetic processes in cancers, can lead to a loss of expression of genes that occurs about 10 times more frequently by transcription silencing than by mutations. As Vogelstein et al. point out, in a colorectal cancer there are usually about 3 to 6 driver mutations and 33 to 66 hitchhiker or passenger mutations. However, in colon tumors compared to adjacent normal-appearing colonic mucosa, there are about 600 to 800 heavily methylated CpG islands in promoters of genes in the tumors while these CpG islands are not methylated in the adjacent mucosa. Manipulation of epigenetic alterations holds great promise for cancer prevention, detection, and therapy. In different types of cancer, a variety of epigenetic mechanisms can be perturbed, such as silencing of tumor suppressor genes and activation of oncogenes by altered CpG island methylation patterns, histone modifications, and dysregulation of DNA binding proteins. Several medications which have epigenetic impact are now used in several of these diseases.
In molecular biology mir-365 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms.
In molecular biology mir-744 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms.
Spindle and kinetochore-associated protein 2 is a protein that in humans is encoded by the SKA2 gene found in chromosome 17. SKA2 is a part of a spindle and kinetochore associated complex also including SKA1 and SKA3 which is responsible for onset of the anaphase in mitosis by regulating chromosomal segregation.
Generally, in progression to cancer, hundreds of genes are silenced or activated. Although silencing of some genes in cancers occurs by mutation, a large proportion of carcinogenic gene silencing is a result of altered DNA methylation. DNA methylation causing silencing in cancer typically occurs at multiple CpG sites in the CpG islands that are present in the promoters of protein coding genes.
DNA methylation in cancer plays a variety of roles, helping to change the healthy regulation of gene expression to a disease pattern.
NamiRNAs are a type of miRNAs present in the nucleus, which can activate [[gene expression]] by binding to the enhancer, and therefore were named nuclear activating miRNAs (NamiRNAs), such as miR-24-1 and miR-26. These miRNAs loci are enriched with epigenetic markers that display enhancer activity like histone H3K27ac, P300/CBP, and DNaseI high-sensitivity loci. These NamiRNAs are able to activate the related enhancers and co-work with them to up-regulate the expression of neighboring genes. NamiRNAs are able to promote global gene transcription by binding their targeted enhancers in whole genome level.
References
- 1 2 3 4 5 Li LC, Okino ST, Zhao H, Pookot D, Place RF, Urakami S, Enokida H, Dahiya R (November 2006). "Small dsRNAs induce transcriptional activation in human cells". Proceedings of the National Academy of Sciences of the United States of America. 103 (46): 17337–42. Bibcode:2006PNAS..10317337L. doi: 10.1073/pnas.0607015103 . PMC 1859931 . PMID 17085592.[ non-primary source needed ]
- ↑ Li, Longcheng; Dahiya, Rajvir. "Small Activating RNA Molecules and Methods of Use." U.S. Patent US 8,877,721 filed October 1, 2004, and issued November 4, 2014.
- ↑ Janowski BA, Younger ST, Hardy DB, Ram R, Huffman KE, Corey DR (March 2007). "Activating gene expression in mammalian cells with promoter-targeted duplex RNAs". Nature Chemical Biology. 3 (3): 166–73. doi:10.1038/nchembio860. PMID 17259978.
- 1 2 3 Turunen MP, Lehtola T, Heinonen SE, Assefa GS, Korpisalo P, Girnary R, Glass CK, Väisänen S, Ylä-Herttuala S (September 2009). "Efficient regulation of VEGF expression by promoter-targeted lentiviral shRNAs based on epigenetic mechanism: a novel example of epigenetherapy". Circulation Research. 105 (6): 604–9. doi: 10.1161/CIRCRESAHA.109.200774 . PMID 19696410.
- ↑ Huang V, Qin Y, Wang J, Wang X, Place RF, Lin G, Lue TF, Li LC (January 2010). Jin D (ed.). "RNAa is conserved in mammalian cells". PLOS ONE. 5 (1): e8848. Bibcode:2010PLoSO...5.8848H. doi: 10.1371/journal.pone.0008848 . PMC 2809750 . PMID 20107511.
- 1 2 Matsui M, Sakurai F, Elbashir S, Foster DJ, Manoharan M, Corey DR (December 2010). "Activation of LDL receptor expression by small RNAs complementary to a noncoding transcript that overlaps the LDLR promoter". Chemistry & Biology. 17 (12): 1344–55. doi:10.1016/j.chembiol.2010.10.009. PMC 3071588 . PMID 21168770.
- ↑ Shibuya K, Fukushima S, Takatsuji H (February 2009). "RNA-directed DNA methylation induces transcriptional activation in plants". Proceedings of the National Academy of Sciences of the United States of America. 106 (5): 1660–5. Bibcode:2009PNAS..106.1660S. doi: 10.1073/pnas.0809294106 . PMC 2629447 . PMID 19164525.
- 1 2 Seth M, Shirayama M, Gu W, Ishidate T, Conte D, Mello CC (December 2013). "The C. elegans CSR-1 argonaute pathway counteracts epigenetic silencing to promote germline gene expression". Developmental Cell. 27 (6): 656–63. doi:10.1016/j.devcel.2013.11.014. PMC 3954781 . PMID 24360782.
- 1 2 3 Turner MJ, Jiao AL, Slack FJ (Jan 7, 2014). "Autoregulation of lin-4 microRNA transcription by RNA activation (RNAa) in C. elegans". Cell Cycle. 13 (5): 772–81. doi:10.4161/cc.27679. PMC 3979913 . PMID 24398561.
- ↑ Yue X, Schwartz JC, Chu Y, Younger ST, Gagnon KT, Elbashir S, Janowski BA, Corey DR (August 2010). "Transcriptional regulation by small RNAs at sequences downstream from 3' gene termini". Nature Chemical Biology. 6 (8): 621–9. doi:10.1038/nchembio.400. PMC 3909968 . PMID 20581822.
- 1 2 Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R (February 2008). "MicroRNA-373 induces expression of genes with complementary promoter sequences". Proceedings of the National Academy of Sciences of the United States of America. 105 (5): 1608–13. Bibcode:2008PNAS..105.1608P. doi: 10.1073/pnas.0707594105 . PMC 2234192 . PMID 18227514.[ non-primary source needed ]
- 1 2 Huang V, Place RF, Portnoy V, Wang J, Qi Z, Jia Z, Yu A, Shuman M, Yu J, Li LC (February 2012). "Upregulation of Cyclin B1 by miRNA and its implications in cancer". Nucleic Acids Research. 40 (4): 1695–707. doi:10.1093/nar/gkr934. PMC 3287204 . PMID 22053081.[ non-primary source needed ]
- 1 2 Chu Y, Yue X, Younger ST, Janowski BA, Corey DR (November 2010). "Involvement of argonaute proteins in gene silencing and activation by RNAs complementary to a non-coding transcript at the progesterone receptor promoter". Nucleic Acids Research. 38 (21): 7736–48. doi:10.1093/nar/gkq648. PMC 2995069 . PMID 20675357.
- ↑ Li, Long-Cheng (2008). "Small RNA-mediated gene activation". In Morris, Kevin V (ed.). RNA and the Regulation of Gene Expression: A Hidden Layer of Complexity. Caister Academic Press. pp. 189–99. ISBN 978-1-904455-25-7.
- 1 2 Portnoy V, Huang V, Place RF, Li LC (2011). "Small RNA and transcriptional upregulation". Wiley Interdisciplinary Reviews: RNA. 2 (5): 748–60. doi:10.1002/wrna.90. PMC 3154074 . PMID 21823233.
- ↑ Meng X, Jiang Q, Chang N, Wang X, Liu C, Xiong J, Cao H, Liang Z (March 2016). "Small activating RNA binds to the genomic target site in a seed-region-dependent manner". Nucleic Acids Research. 44 (5): 2274–82. doi:10.1093/nar/gkw076. PMC 4797303 . PMID 26873922.
- ↑ Portnoy V, Lin SH, Li KH, Burlingame A, Hu ZH, Li H, Li LC (March 2016). "saRNA-guided Ago2 targets the RITA complex to promoters to stimulate transcription". Cell Research. 26 (3): 320–35. doi:10.1038/cr.2016.22. PMC 4783471 . PMID 26902284.
- ↑ Voutila J, Reebye V, Roberts TC, Protopapa P, Andrikakou P, Blakey DC, Habib R, Huber H, Saetrom P, Rossi JJ, Habib NA (December 2017). "Development and Mechanism of Small Activating RNA Targeting CEBPA, a Novel Therapeutic in Clinical Trials for Liver Cancer". Molecular Therapy. 25 (12): 2705–2714. doi:10.1016/j.ymthe.2017.07.018. PMC 5768526 . PMID 28882451.
- ↑ Matsui M, Chu Y, Zhang H, Gagnon KT, Shaikh S, Kuchimanchi S, Manoharan M, Corey DR, Janowski BA (December 2013). "Promoter RNA links transcriptional regulation of inflammatory pathway genes". Nucleic Acids Research. 41 (22): 10086–109. doi:10.1093/nar/gkt777. PMC 3905862 . PMID 23999091.
- ↑ Dharap A, Pokrzywa C, Murali S, Pandi G, Vemuganti R (2013). "MicroRNA miR-324-3p induces promoter-mediated expression of RelA gene". PLOS ONE. 8 (11): e79467. Bibcode:2013PLoSO...879467D. doi: 10.1371/journal.pone.0079467 . PMC 3827167 . PMID 24265774.
- 1 2 3 Chaluvally-Raghavan P, Jeong KJ, Pradeep S, Silva AM, Yu S, Liu W, Moss T, Rodriguez-Aguayo C, Zhang D, Ram P, Liu J, Lu Y, Lopez-Berestein G, Calin GA, Sood AK, Mills GB (May 2016). "Direct Upregulation of STAT3 by MicroRNA-551b-3p Deregulates Growth and Metastasis of Ovarian Cancer". Cell Reports. 15 (7): 1493–1504. doi:10.1016/j.celrep.2016.04.034. PMC 4914391 . PMID 27160903.
- ↑ Li S, Wang C, Yu X, Wu H, Hu J, Wang S, Ye Z (January 2017). "miR-3619-5p inhibits prostate cancer cell growth by activating CDKN1A expression". Oncology Reports. 37 (1): 241–248. doi: 10.3892/or.2016.5250 . PMID 27878260.
- ↑ Xiao M, Li J, Li W, Wang Y, Wu F, Xi Y, Zhang L, Ding C, Luo H, Li Y, Peng L, Zhao L, Peng S, Xiao Y, Dong S, Cao J, Yu W (October 2017). "MicroRNAs activate gene transcription epigenetically as an enhancer trigger". RNA Biology. 14 (10): 1326–1334. doi:10.1080/15476286.2015.1112487. PMC 5711461 . PMID 26853707.
- ↑ Vaschetto LM (April 2018). "miRNA activation is an endogenous gene expression pathway". RNA Biology. 15 (6): 826–828. doi:10.1080/15476286.2018.1451722. PMC 6152443 . PMID 29537927.
- ↑ Conine CC, Moresco JJ, Gu W, Shirayama M, Conte D, Yates JR, Mello CC (December 2013). "Argonautes promote male fertility and provide a paternal memory of germline gene expression in C. elegans". Cell. 155 (7): 1532–44. doi:10.1016/j.cell.2013.11.032. PMC 3924572 . PMID 24360276.
- ↑ Wedeles CJ, Wu MZ, Claycomb JM (December 2013). "Protection of germline gene expression by the C. elegans Argonaute CSR-1". Developmental Cell. 27 (6): 664–71. doi: 10.1016/j.devcel.2013.11.016 . PMID 24360783.
- ↑ Paugh SW, Coss DR, Bao J, Laudermilk LT, Grace CR, Ferreira AM, Waddell MB, Ridout G, Naeve D, Leuze M, LoCascio PF, Panetta JC, Wilkinson MR, Pui CH, Naeve CW, Uberbacher EC, Bonten EJ, Evans WE (February 2016). "MicroRNAs Form Triplexes with Double Stranded DNA at Sequence-Specific Binding Sites; a Eukaryotic Mechanism via which microRNAs Could Directly Alter Gene Expression". PLOS Computational Biology. 12 (2): e1004744. Bibcode:2016PLSCB..12E4744P. doi:10.1371/journal.pcbi.1004744. PMC 4742280 . PMID 26844769.
- ↑ Huang V, Zheng J, Qi Z, Wang J, Place RF, Yu J, Li H, Li LC (2013). "Ago1 Interacts with RNA polymerase II and binds to the promoters of actively transcribed genes in human cancer cells". PLOS Genetics. 9 (9): e1003821. doi:10.1371/journal.pgen.1003821. PMC 3784563 . PMID 24086155.
- ↑ Wang J, Place RF, Huang V, Wang X, Noonan EJ, Magyar CE, Huang J, Li LC (December 2010). "Prognostic value and function of KLF4 in prostate cancer: RNAa and vector-mediated overexpression identify KLF4 as an inhibitor of tumor cell growth and migration". Cancer Research. 70 (24): 10182–91. doi:10.1158/0008-5472.CAN-10-2414. PMC 3076047 . PMID 21159640.
- ↑ Chen R, Wang T, Rao K, Yang J, Zhang S, Wang S, Liu J, Ye Z (October 2011). "Up-regulation of VEGF by small activator RNA in human corpus cavernosum smooth muscle cells". The Journal of Sexual Medicine. 8 (10): 2773–80. doi:10.1111/j.1743-6109.2011.02412.x. PMID 21819543.
- ↑ Kang MR, Yang G, Place RF, Charisse K, Epstein-Barash H, Manoharan M, Li LC (October 2012). "Intravesical delivery of small activating RNA formulated into lipid nanoparticles inhibits orthotopic bladder tumor growth". Cancer Research. 72 (19): 5069–79. doi: 10.1158/0008-5472.can-12-1871 . PMID 22869584.
- ↑ Place RF, Wang J, Noonan EJ, Meyers R, Manoharan M, Charisse K, Duncan R, Huang V, Wang X, Li LC (March 2012). "Formulation of Small Activating RNA Into Lipidoid Nanoparticles Inhibits Xenograft Prostate Tumor Growth by Inducing p21 Expression". Molecular Therapy. Nucleic Acids. 1 (3): e15. doi:10.1038/mtna.2012.5. PMC 3381590 . PMID 23343884.
- ↑ Yoon S, Huang KW, Reebye V, Mintz P, Tien YW, Lai HS, Sætrom P, Reccia I, Swiderski P, Armstrong B, Jozwiak A, Spalding D, Jiao L, Habib N, Rossi JJ (June 2016). "Targeted Delivery of C/EBPα -saRNA by Pancreatic Ductal Adenocarcinoma-specific RNA Aptamers Inhibits Tumor Growth In Vivo". Molecular Therapy. 24 (6): 1106–1116. doi:10.1038/mt.2016.60. PMC 4923325 . PMID 26983359.
- ↑ Huan H, Wen X, Chen X, Wu L, Liu W, Habib NA, Bie P, Xia F (2016-01-01). "C/EBPα Short-Activating RNA Suppresses Metastasis of Hepatocellular Carcinoma through Inhibiting EGFR/β-Catenin Signaling Mediated EMT". PLOS ONE. 11 (4): e0153117. Bibcode:2016PLoSO..1153117H. doi: 10.1371/journal.pone.0153117 . PMC 4822802 . PMID 27050434.
- ↑ Li C, Jiang W, Hu Q, Li LC, Dong L, Chen R, Zhang Y, Tang Y, Thrasher JB, Liu CB, Li B (April 2016). "Enhancing DPYSL3 gene expression via a promoter-targeted small activating RNA approach suppresses cancer cell motility and metastasis". Oncotarget. 7 (16): 22893–910. doi:10.18632/oncotarget.8290. PMC 5008410 . PMID 27014974.
- ↑ Reebye V, Huang KW, Lin V, Jarvis S, Cutilas P, Dorman S, Ciriello S, Andrikakou P, Voutila J, Saetrom P, Mintz PJ, Reccia I, Rossi JJ, Huber H, Habib R, Kostomitsopoulos N, Blakey DC, Habib NA (June 2018). "Gene activation of CEBPA using saRNA: preclinical studies of the first in human saRNA drug candidate for liver cancer". Oncogene. 37 (24): 3216–3228. doi:10.1038/s41388-018-0126-2. PMC 6013054 . PMID 29511346.
- ↑ "MiNA Therapeutics Announces Initiation of Phase I Clinical Study of MTL-CEBPA in Patients with Liver Cancer | Business Wire". www.businesswire.com. 2 June 2016. Retrieved 2016-06-06.
- ↑ "First-in-Human Safety and Tolerability Study of MTL-CEBPA in Patients With Advanced Liver Cancer - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2016-06-06.