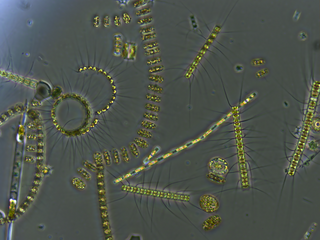
Phytoplankton are the autotrophic (self-feeding) components of the plankton community and a key part of ocean and freshwater ecosystems. The name comes from the Greek words φυτόν, meaning 'plant', and, meaning 'wanderer' or 'drifter'.

Eutrophication is a general term describing a process in which nutrients accumulate in a body of water, resulting in an increased growth of microorganisms that may deplete the water of oxygen. Although eutrophication is a natural process and, manmade or cultural eutrophication is far more common and is a rapid process caused by a variety of polluting inputs including poorly treated sewage, industrial wastewater, and fertilizer runoff. Such nutrient pollution usually causes algal blooms and bacterial growth, resulting in the depletion of dissolved oxygen in water and causing substantial environmental degradation.

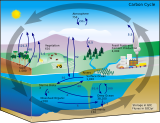
The biological pump (or ocean carbon biological pump or marine biological carbon pump) is the ocean's biologically driven sequestration of carbon from the atmosphere and land runoff to the ocean interior and seafloor sediments. In other words, it is a biologically mediated process which results in the sequestering of carbon in the deep ocean away from the atmosphere and the land. The biological pump is the biological component of the "marine carbon pump" which contains both a physical and biological component. It is the part of the broader oceanic carbon cycle responsible for the cycling of organic matter formed mainly by phytoplankton during photosynthesis (soft-tissue pump), as well as the cycling of calcium carbonate (CaCO3) formed into shells by certain organisms such as plankton and mollusks (carbonate pump).
A limiting factor is a variable of a system that causes a noticeable change in output or another measure of a type of system. The limiting factor is in a pyramid shape of organisms going up from the producers to consumers and so on. A factor not limiting over a certain domain of starting conditions may yet be limiting over another domain of starting conditions, including that of the factor.
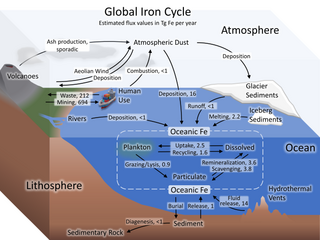
The iron cycle (Fe) is the biogeochemical cycle of iron through the atmosphere, hydrosphere, biosphere and lithosphere. While Fe is highly abundant in the Earth's crust, it is less common in oxygenated surface waters. Iron is a key micronutrient in primary productivity, and a limiting nutrient in the Southern ocean, eastern equatorial Pacific, and the subarctic Pacific referred to as High-Nutrient, Low-Chlorophyll (HNLC) regions of the ocean.
High-nutrient, low-chlorophyll (HNLC) regions are regions of the ocean where the abundance of phytoplankton is low and fairly constant despite the availability of macronutrients. Phytoplankton rely on a suite of nutrients for cellular function. Macronutrients are generally available in higher quantities in surface ocean waters, and are the typical components of common garden fertilizers. Micronutrients are generally available in lower quantities and include trace metals. Macronutrients are typically available in millimolar concentrations, while micronutrients are generally available in micro- to nanomolar concentrations. In general, nitrogen tends to be a limiting ocean nutrient, but in HNLC regions it is never significantly depleted. Instead, these regions tend to be limited by low concentrations of metabolizable iron. Iron is a critical phytoplankton micronutrient necessary for enzyme catalysis and electron transport.

In oceanic biogeochemistry, the f-ratio is the fraction of total primary production fuelled by nitrate. The ratio was originally defined by Richard Eppley and Bruce Peterson in one of the first papers estimating global oceanic production. This fraction was originally believed significant because it appeared to directly relate to the sinking (export) flux of organic marine snow from the surface ocean by the biological pump. However, this interpretation relied on the assumption of a strong depth-partitioning of a parallel process, nitrification, that more recent measurements has questioned.

Ecological stoichiometry considers how the balance of energy and elements influences living systems. Similar to chemical stoichiometry, ecological stoichiometry is founded on constraints of mass balance as they apply to organisms and their interactions in ecosystems. Specifically, how does the balance of energy and elements affect and how is this balance affected by organisms and their interactions. Concepts of ecological stoichiometry have a long history in ecology with early references to the constraints of mass balance made by Liebig, Lotka, and Redfield. These earlier concepts have been extended to explicitly link the elemental physiology of organisms to their food web interactions and ecosystem function.

Ocean fertilization or ocean nourishment is a type of technology for carbon dioxide removal from the ocean based on the purposeful introduction of plant nutrients to the upper ocean to increase marine food production and to remove carbon dioxide from the atmosphere. Ocean nutrient fertilization, for example iron fertilization, could stimulate photosynthesis in phytoplankton. The phytoplankton would convert the ocean's dissolved carbon dioxide into carbohydrate, some of which would sink into the deeper ocean before oxidizing. More than a dozen open-sea experiments confirmed that adding iron to the ocean increases photosynthesis in phytoplankton by up to 30 times.

The phosphorus cycle is the biogeochemical cycle that describes the movement of phosphorus through the lithosphere, hydrosphere, and biosphere. Unlike many other biogeochemical cycles, the atmosphere does not play a significant role in the movement of phosphorus, because phosphorus and phosphorus-based compounds are usually solids at the typical ranges of temperature and pressure found on Earth. The production of phosphine gas occurs in only specialized, local conditions. Therefore, the phosphorus cycle should be viewed from whole Earth system and then specifically focused on the cycle in terrestrial and aquatic systems.
The deep chlorophyll maximum (DCM), also called the subsurface chlorophyll maximum, is the region below the surface of water with the maximum concentration of chlorophyll. The DCM generally exists at the same depth as the nutricline, the region of the ocean where the greatest change in the nutrient concentration occurs with depth.

Bacterioplankton refers to the bacterial component of the plankton that drifts in the water column. The name comes from the Ancient Greek word πλανκτος, meaning "wanderer" or "drifter", and bacterium, a Latin term coined in the 19th century by Christian Gottfried Ehrenberg. They are found in both seawater and freshwater.

The North Pacific Subtropical Gyre (NPSG) is the largest contiguous ecosystem on earth. In oceanography, a subtropical gyre is a ring-like system of ocean currents rotating clockwise in the Northern Hemisphere and counterclockwise in the Southern Hemisphere caused by the Coriolis Effect. They generally form in large open ocean areas that lie between land masses.

A planktivore is an aquatic organism that feeds on planktonic food, including zooplankton and phytoplankton. Planktivorous organisms encompass a range of some of the planet's smallest to largest multicellular animals in both the present day and in the past billion years; basking sharks and copepods are just two examples of giant and microscopic organisms that feed upon plankton. Planktivory can be an important mechanism of top-down control that contributes to trophic cascades in aquatic and marine systems. There is a tremendous diversity of feeding strategies and behaviors that planktivores utilize to capture prey. Some planktivores utilize tides and currents to migrate between estuaries and coastal waters; other aquatic planktivores reside in lakes or reservoirs where diverse assemblages of plankton are present, or migrate vertically in the water column searching for prey. Planktivore populations can impact the abundance and community composition of planktonic species through their predation pressure, and planktivore migrations facilitate nutrient transport between benthic and pelagic habitats.

Marine biogeochemical cycles are biogeochemical cycles that occur within marine environments, that is, in the saltwater of seas or oceans or the brackish water of coastal estuaries. These biogeochemical cycles are the pathways chemical substances and elements move through within the marine environment. In addition, substances and elements can be imported into or exported from the marine environment. These imports and exports can occur as exchanges with the atmosphere above, the ocean floor below, or as runoff from the land.
The Southern Ocean Carbon and Climate Observations and Modeling (SOCCOM) project is a large scale National Science Foundation funded research project based at Princeton University that started in September 2014. The project aims to increase the understanding of the Southern Ocean and the role it plays in factors such as climate, as well as educate new scientists with oceanic observation.
Nutrient cycling in the Columbia River Basin involves the transport of nutrients through the system, as well as transformations from among dissolved, solid, and gaseous phases, depending on the element. The elements that constitute important nutrient cycles include macronutrients such as nitrogen, silicate, phosphorus, and micronutrients, which are found in trace amounts, such as iron. Their cycling within a system is controlled by many biological, chemical, and physical processes.

The viral shunt is a mechanism that prevents marine microbial particulate organic matter (POM) from migrating up trophic levels by recycling them into dissolved organic matter (DOM), which can be readily taken up by microorganisms. The DOM recycled by the viral shunt pathway is comparable to the amount generated by the other main sources of marine DOM.

Marine primary production is the chemical synthesis in the ocean of organic compounds from atmospheric or dissolved carbon dioxide. It principally occurs through the process of photosynthesis, which uses light as its source of energy, but it also occurs through chemosynthesis, which uses the oxidation or reduction of inorganic chemical compounds as its source of energy. Almost all life on Earth relies directly or indirectly on primary production. The organisms responsible for primary production are called primary producers or autotrophs.
Low-nutrient, low-chlorophyll (LNLC)regions are aquatic zones that are low in nutrients and consequently have low rate of primary production, as indicated by low chlorophyll concentrations. These regions can be described as oligotrophic, and about 75% of the world's oceans encompass LNLC regions. A majority of LNLC regions are associated with subtropical gyres but are also present in areas of the Mediterranean Sea, and some inland lakes. Physical processes limit nutrient availability in LNLC regions, which favors nutrient recycling in the photic zone and selects for smaller phytoplankton species. LNLC regions are generally not found near coasts, owing to the fact that coastal areas receive more nutrients from terrestrial sources and upwelling. In marine systems, seasonal and decadal variability of primary productivity in LNLC regions is driven in part by large-scale climatic regimes leading to important effects on the global carbon cycle and the oceanic carbon cycle.