
Ticks are parasitic arachnids that are part of the mite superorder Parasitiformes. Adult ticks are approximately 3 to 5 mm in length depending on age, sex, species, and "fullness". Ticks are external parasites, living by feeding on the blood of mammals, birds, and sometimes reptiles and amphibians. The timing of the origin of ticks is uncertain, though the oldest known tick fossils are from the Cretaceous period, around 100 million years old. Ticks are widely distributed around the world, especially in warm, humid climates.

The Ixodidae are the family of hard ticks or scale ticks, one of the three families of ticks, consisting of over 700 species. They are known as 'hard ticks' because they have a scutum or hard shield, which the other major family of ticks, the 'soft ticks' (Argasidae), lack. They are ectoparasites of a wide range of host species, and some are vectors of pathogens that can cause human disease.

Babesia, also called Nuttallia, is an apicomplexan parasite that infects red blood cells and is transmitted by ticks. Originally discovered by the Romanian bacteriologist Victor Babeș in 1888, over 100 species of Babesia have since been identified.
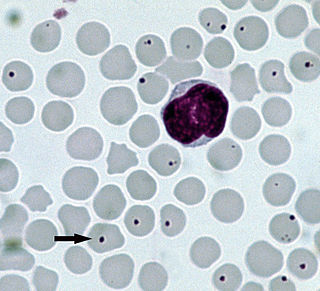
Anaplasmosis is a tick-borne disease affecting ruminants, dogs, and horses, and is caused by Anaplasma bacteria. Anaplasmosis is an infectious but not contagious disease. Anaplasmosis can be transmitted through mechanical and biological vector processes. Anaplasmosis can also be referred to as "yellow bag" or "yellow fever" because the infected animal can develop a jaundiced look. Other signs of infection include weight loss, diarrhea, paleness of the skin, aggressive behavior, and high fever.
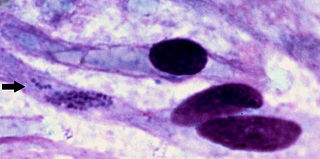
Heartwater is a tick-borne rickettsial disease. The name is derived from the fact that fluid can collect around the heart or in the lungs of infected animals. It is caused by Ehrlichia ruminantium —an intracellular Gram-negative coccal bacterium. The disease is spread by various Amblyomma ticks, and has a large economic impact on cattle production in affected areas. There are four documented manifestations of the disease, these are acute, peracute, subacute, and a mild form known as heartwater fever. There are reports of zoonotic infections of humans by E. ruminantium, similar to other Ehrlichia species, such as those that cause human ehrlichiosis.

Rhipicephalus sanguineus, commonly called the brown dog tick, kennel tick, or pantropical dog tick, is a species of tick found worldwide, but more commonly in warmer climates. This species is unusual among ticks in that its entire lifecycle can be completed indoors. The brown dog tick is easily recognized by its reddish-brown color, elongated body shape, and hexagonal basis capituli. Adults are 2.28 to 3.18 mm in length and 1.11 to 1.68 mm in width. They do not have ornamentation on their backs.

Dermacentor is a genus of ticks in the family Ixodidae, the hard ticks. The genus has a cosmopolitan distribution, with native species on all continents except Australia. Most are found in North America.

Rhipicephalus is a genus of ticks in the family Ixodidae, the hard ticks, consisting of about 74 or 75 species. Most are native to tropical Africa.

Ixodes pacificus, the western black-legged tick, is a species of parasitic tick found on the western coast of North America. I. pacificus is a member of the family Ixodidae. It is the principal vector of Lyme disease in that region. I. pacificus typically feeds on lizards and small mammals therefore its rate of transmission of Lyme disease to humans is around 1% of adults. It is an ectoparasite that attaches itself to the outside of its host and feeds on the host's blood. It can have a heteroxenous lifestyle or monoxenous life cycle depending on how many hosts it feeds on in each cycle. I. pacificus has a four stage life cycle that takes around 3 years to complete. These stages include egg, larva, nymph, and adult. They prefer dense woodland habitats or areas of brush and tall grass.

Babesia bovis is an Apicomplexan single-celled parasite of cattle which occasionally infects humans. The disease it and other members of the genus Babesia cause is a hemolytic anemia known as babesiosis and colloquially called Texas cattle fever, redwater or piroplasmosis. It is transmitted by bites from infected larval ticks of the order Ixodida. It was eradicated from the United States by 1943, but is still present in Mexico and much of the world's tropics. The chief vector of Babesia species is the southern cattle fever tick Rhipicephalus microplus.

Ticks of domestic animals directly cause poor health and loss of production to their hosts. Ticks also transmit numerous kinds of viruses, bacteria, and protozoa between domestic animals. These microbes cause diseases which can be severely debilitating or fatal to domestic animals, and may also affect humans. Ticks are especially important to domestic animals in tropical and subtropical countries, where the warm climate enables many species to flourish. Also, the large populations of wild animals in warm countries provide a reservoir of ticks and infective microbes that spread to domestic animals. Farmers of livestock animals use many methods to control ticks, and related treatments are used to reduce infestation of companion animals.
Anaplasma bovis is gram negative, obligate intracellular organism, which can be found in wild and domestic ruminants, and potentially a wide variety of other species. It is one of the last species of the Family Anaplasmaceae to be formally described. It preferentially infects host monocytes, and is often diagnosed via blood smears, PCR, and ELISA. A. bovis is not currently considered zoonotic, and does not frequently cause serious clinical disease in its host. This organism is transmitted by tick vectors, so tick bite prevention is the mainstay of A. bovis control, although clinical infections can be treated with tetracyclines. This organism has a global distribution, with infections noted in many areas, including Korea, Japan, Europe, Brazil, Africa, and North America.

Amblyomma variegatum, commonly known as the tropical bont tick, is a species of tick of the genus Amblyomma endemic to Africa. It has spread from its centre of origin to several countries, including the Caribbean islands, where it is known as the Senegalese tick and the Antigua gold tick. They are vividly coloured and have a substantial impact on livestock, primarily through their transmission of diseases. They are three-host hard ticks that have been found on a variety of domesticated species such as camels, cattle, goats, sheep, dogs, and various species of wildlife.
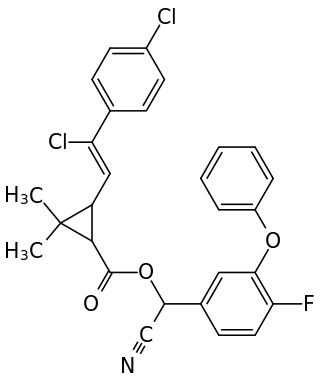
Flumethrin is a pyrethroid insecticide. It is used externally in veterinary medicine against parasitic insects and ticks on cattle, sheep, goats, horses, and dogs, and the treatment of parasitic mites in honeybee colonies.

Mites are small crawling animals related to ticks and spiders. Most mites are free-living and harmless. Other mites are parasitic, and those that infest livestock animals cause many diseases that are widespread, reduce production and profit for farmers, and are expensive to control.

Hyalomma dromedarii is a species of hard-bodied ticks belonging to the family Ixodidae.
Haemaphysalis bispinosa is a hard-bodied tick of the genus Haemaphysalis. It is found in India, Sri Lanka, Myanmar, Pakistan, Nepal, Australia, and Indonesia. It is an obligate ectoparasite of mammals. It is a potential vector of Kyasanur Forest disease virus. These ticks was found parasitized by a chalcid Hunterellus sagarensis in these diseased areas.

Rhipicephalus annulatus, the cattle tick, is a hard-bodied tick of the genus Rhipicephalus. It is also known as North American cattle tick, North American Texas fever tick, and Texas fever tick.

The zebra tick or yellow back tick is a species of hard tick. It is common in the Horn of Africa, with a habitat of the Rift Valley and eastward. It feeds upon a wide variety of species, including livestock, wild mammals, and humans, and can be a vector for various pathogens. The adult male has a distinctive black and ivory ornamentation on its scutum.














