Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. Autoxidation leads to degradation of organic compounds, including living matter. Antioxidants are frequently added to industrial products, such as polymers, fuels, and lubricants, to extend their usable lifetimes. Food are also treated with antioxidants to forestall spoilage, in particular the rancidification of oils and fats. In cells, antioxidants such as glutathione, mycothiol or bacillithiol, and enzyme systems like superoxide dismutase, can prevent damage from oxidative stress.
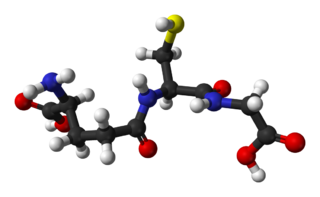
Glutathione is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, peroxides, lipid peroxides, and heavy metals. It is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and cysteine. The carboxyl group of the cysteine residue is attached by normal peptide linkage to glycine.
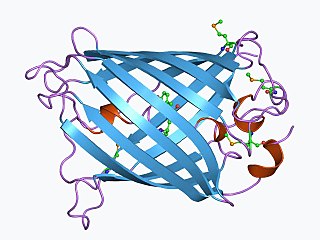
The green fluorescent protein (GFP) is a protein that exhibits bright green fluorescence when exposed to light in the blue to ultraviolet range. The label GFP traditionally refers to the protein first isolated from the jellyfish Aequorea victoria and is sometimes called avGFP. However, GFPs have been found in other organisms including corals, sea anemones, zoanithids, copepods and lancelets.
In molecular biology and biotechnology, a fluorescent tag, also known as a fluorescent label or fluorescent probe, is a molecule that is attached chemically to aid in the detection of a biomolecule such as a protein, antibody, or amino acid. Generally, fluorescent tagging, or labeling, uses a reactive derivative of a fluorescent molecule known as a fluorophore. The fluorophore selectively binds to a specific region or functional group on the target molecule and can be attached chemically or biologically. Various labeling techniques such as enzymatic labeling, protein labeling, and genetic labeling are widely utilized. Ethidium bromide, fluorescein and green fluorescent protein are common tags. The most commonly labelled molecules are antibodies, proteins, amino acids and peptides which are then used as specific probes for detection of a particular target.
A biosensor is an analytical device, used for the detection of a chemical substance, that combines a biological component with a physicochemical detector. The sensitive biological element, e.g. tissue, microorganisms, organelles, cell receptors, enzymes, antibodies, nucleic acids, etc., is a biologically derived material or biomimetic component that interacts with, binds with, or recognizes the analyte under study. The biologically sensitive elements can also be created by biological engineering. The transducer or the detector element, which transforms one signal into another one, works in a physicochemical way: optical, piezoelectric, electrochemical, electrochemiluminescence etc., resulting from the interaction of the analyte with the biological element, to easily measure and quantify. The biosensor reader device connects with the associated electronics or signal processors that are primarily responsible for the display of the results in a user-friendly way. This sometimes accounts for the most expensive part of the sensor device, however it is possible to generate a user friendly display that includes transducer and sensitive element. The readers are usually custom-designed and manufactured to suit the different working principles of biosensors.

Protein disulfide isomerase, or PDI, is an enzyme in the endoplasmic reticulum (ER) in eukaryotes and the periplasm of bacteria that catalyzes the formation and breakage of disulfide bonds between cysteine residues within proteins as they fold. This allows proteins to quickly find the correct arrangement of disulfide bonds in their fully folded state, and therefore the enzyme acts to catalyze protein folding.
Thioredoxin reductases are enzymes that reduce thioredoxin (Trx). Two classes of thioredoxin reductase have been identified: one class in bacteria and some eukaryotes and one in animals. In bacteria TrxR also catalyzes the reduction of glutaredoxin like proteins known as NrdH. Both classes are flavoproteins which function as homodimers. Each monomer contains a FAD prosthetic group, a NADPH binding domain, and an active site containing a redox-active disulfide bond.

Glutathione disulfide (GSSG) is a disulfide derived from two glutathione molecules.

Glutaredoxins are small redox enzymes of approximately one hundred amino-acid residues that use glutathione as a cofactor. In humans this oxidation repair enzyme is also known to participate in many cellular functions, including redox signaling and regulation of glucose metabolism. Glutaredoxins are oxidized by substrates, and reduced non-enzymatically by glutathione. In contrast to thioredoxins, which are reduced by thioredoxin reductase, no oxidoreductase exists that specifically reduces glutaredoxins. Instead, glutaredoxins are reduced by the oxidation of glutathione. Reduced glutathione is then regenerated by glutathione reductase. Together these components compose the glutathione system.

Nuclear factor erythroid 2-related factor 2 (NRF2), also known as nuclear factor erythroid-derived 2-like 2, is a transcription factor that in humans is encoded by the NFE2L2 gene. NRF2 is a basic leucine zipper (bZIP) protein that may regulate the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation, according to preliminary research. In vitro, NRF2 binds to antioxidant response elements (AREs) in the promoter regions of genes encoding cytoprotective proteins. NRF2 induces the expression of heme oxygenase 1 in vitro leading to an increase in phase II enzymes. NRF2 also inhibits the NLRP3 inflammasome.
Glutamate–cysteine ligase (GCL) EC 6.3.2.2), previously known as γ-glutamylcysteine synthetase (GCS), is the first enzyme of the cellular glutathione (GSH) biosynthetic pathway that catalyzes the chemical reaction:
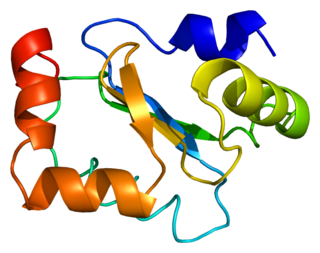
Glutaredoxin-1 is a protein that in humans is encoded by the GLRX gene.

Glutaredoxin 2 (GLRX2) is an enzyme that in humans encoded by the GLRX2 gene. GLRX2, also known as GRX2, is a glutaredoxin family protein and a thiol-disulfide oxidoreductase that maintains cellular thiol homeostasis. This gene consists of four exons and three introns, spanned 10 kilobase pairs, and localized to chromosome 1q31.2–31.3.

γ-L-Glutamyl-L-cysteine, also known as γ-glutamylcysteine (GGC), is a dipeptide found in animals, plants, fungi, some bacteria, and archaea. It has a relatively unusual γ-bond between the constituent amino acids, L-glutamic acid and L-cysteine and is a key intermediate in the γ-glutamyl cycle first described by Meister in the 1970s. It is the most immediate precursor to the antioxidant glutathione.

GCaMP is a genetically encoded calcium indicator (GECI) initially developed in 2001 by Junichi Nakai. It is a synthetic fusion of green fluorescent protein (GFP), calmodulin (CaM), and M13, a peptide sequence from myosin light-chain kinase. When bound to Ca2+, GCaMP fluoresces green with a peak excitation wavelength of 480 nm and a peak emission wavelength of 510 nm. It is used in biological research to measure intracellular Ca2+ levels both in vitro and in vivo using virally transfected or transgenic cell and animal lines. The genetic sequence encoding GCaMP can be inserted under the control of promoters exclusive to certain cell types, allowing for cell-type specific expression of GCaMP. Since Ca2+ is a second messenger that contributes to many cellular mechanisms and signaling pathways, GCaMP allows researchers to quantify the activity of Ca2+-based mechanisms and study the role of Ca2+ ions in biological processes of interest.
Calcium imaging is a microscopy technique to optically measure the calcium (Ca2+) status of an isolated cell, tissue or medium. Calcium imaging takes advantage of calcium indicators, fluorescent molecules that respond to the binding of Ca2+ ions by fluorescence properties. Two main classes of calcium indicators exist: chemical indicators and genetically encoded calcium indicators (GECI). This technique has allowed studies of calcium signalling in a wide variety of cell types. In neurons, electrical activity is always accompanied by an influx of Ca2+ ions. Thus, calcium imaging can be used to monitor the electrical activity in hundreds of neurons in cell culture or in living animals, which has made it possible to dissect the function of neuronal circuits.
Thiol oxidoreductases are proteins that redox control by utilizing catalytic cysteine (Cys) residues for oxidation or reduction of their substrates. Examples of such proteins include thioredoxin, thioredoxin reductase, glutathione reductase, glutaredoxin, glutathione peroxidase, and peroxiredoxin.

Small ultra red fluorescent protein (smURFP) is a class of far-red fluorescent protein evolved from a cyanobacterial phycobiliprotein, α-allophycocyanin. Native α-allophycocyanin requires an exogenous protein, known as a lyase, to attach the chromophore, phycocyanobilin. Phycocyanobilin is not present in mammalian cells. smURFP was evolved to covalently attach phycocyanobilin without a lyase and fluoresce, covalently attach biliverdin and fluoresce, blue-shift fluorescence to match the organic fluorophore, Cy5, and not inhibit E. coli growth. smURFP was found after 12 rounds of random mutagenesis and manually screening 10,000,000 bacterial colonies.

Red fluorescent protein (RFP) is a fluorophore that fluoresces red-orange when excited. Several variants have been developed using directed mutagenesis. The original was isolated from Discosoma, and named DsRed. Others are now available that fluoresce orange, red, and far-red.
Optogenetics began with methods to alter neuronal activity with light, using e.g. channelrhodopsins. In a broader sense, optogenetic approaches also include the use of genetically encoded biosensors to monitor the activity of neurons or other cell types by measuring fluorescence or bioluminescence. Genetically encoded calcium indicators (GECIs) are used frequently to monitor neuronal activity, but other cellular parameters such as membrane voltage or second messenger activity can also be recorded optically. The use of optogenetic sensors is not restricted to neuroscience, but plays increasingly important roles in immunology, cardiology and cancer research.













