
Limb–girdle muscular dystrophy (LGMD) is a genetically heterogeneous group of rare muscular dystrophies that share a set of clinical characteristics. It is characterised by progressive muscle wasting which affects predominantly hip and shoulder muscles. LGMD usually has an autosomal pattern of inheritance. It currently has no known cure or treatment.
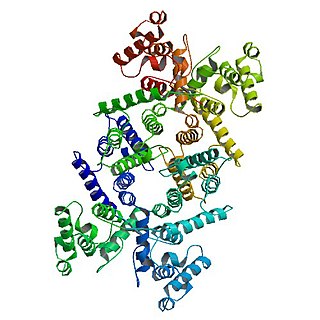
Dystrophin is a rod-shaped cytoplasmic protein, and a vital part of a protein complex that connects the cytoskeleton of a muscle fiber to the surrounding extracellular matrix through the cell membrane. This complex is variously known as the costamere or the dystrophin-associated protein complex (DAPC). Many muscle proteins, such as α-dystrobrevin, syncoilin, synemin, sarcoglycan, dystroglycan, and sarcospan, colocalize with dystrophin at the costamere. It has a molecular weight of 427 kDa

Fukuyama congenital muscular dystrophy (FCMD) is a rare, autosomal recessive form of muscular dystrophy (weakness and breakdown of muscular tissue) mainly described in Japan but also identified in Turkish and Ashkenazi Jewish patients; fifteen cases were first described on 1960 by Dr. Yukio Fukuyama.
The sarcoglycanopathies are a collection of diseases resulting from mutations in any of the five sarcoglycan genes: α, β, γ, δ or ε. The five sarcoglycanopathies are: α-sarcoglycanopathy, LGMD2D; β-sarcoglycanopathy, LGMD2E; γ-sarcoglycanopathy, LGMD2C; δ-sarcoglycanopathy, LGMD2F and ε-sarcoglycanopathy, myoclonic dystonia. The four different sarcoglycan genes encode proteins that form a tetrameric complex at the muscle cell plasma membrane. This complex stabilizes the association of dystrophin with the dystroglycans and contributes to the stability of the plasma membrane cytoskeleton. The four sarcoglycan genes are related to each other structurally and functionally, but each has a distinct chromosome location.
The sarcoglycans are a family of transmembrane proteins involved in the protein complex responsible for connecting the muscle fibre cytoskeleton to the extracellular matrix, preventing damage to the muscle fibre sarcolemma through shearing forces.

Emery–Dreifuss muscular dystrophy (EDMD) is a type of muscular dystrophy, a group of heritable diseases that cause progressive impairment of muscles. EDMD affects muscles used for movement, causing atrophy, weakness and contractures. It almost always affects the heart, causing abnormal rhythms, heart failure, or sudden cardiac death. It is rare, affecting 0.39 per 100,000 people. It is named after Alan Eglin H. Emery and Fritz E. Dreifuss.

Dystroglycan is a protein that in humans is encoded by the DAG1 gene.
The costamere is a structural-functional component of striated muscle cells which connects the sarcomere of the muscle to the cell membrane.

Originally identified as Kirsten ras associated gene (KRAG), sarcospan (SSPN) is a 25-kDa transmembrane protein located in the dystrophin-associated protein complex of skeletal muscle cells, where it is most abundant. It contains four transmembrane spanning helices with both N- and C-terminal domains located intracellularly. Loss of SSPN expression occurs in patients with Duchenne muscular dystrophy. Dystrophin is required for proper localization of SSPN. SSPN is also an essential regulator of Akt signaling pathways. Without SSPN, Akt signaling pathways will be hindered and muscle regeneration will not occur.

Fukutin is a eukaryotic protein necessary for the maintenance of muscle integrity, cortical histogenesis, and normal ocular development. Mutations in the fukutin gene have been shown to result in Fukuyama congenital muscular dystrophy (FCMD) characterised by brain malformation - one of the most common autosomal-recessive disorders in Japan. In humans this protein is encoded by the FCMD gene, located on chromosome 9q31. Human fukutin exhibits a length of 461 amino acids and a predicted molecular mass of 53.7 kDa.
Calpain-3 is a protein that in humans is encoded by the CAPN3 gene.
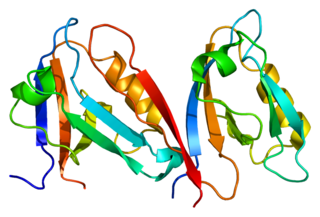
Alpha-1-syntrophin is a protein that in humans is encoded by the SNTA1 gene. Alpha-1 syntrophin is a signal transducing adaptor protein and serves as a scaffold for various signaling molecules. Alpha-1 syntrophin contains a PDZ domain, two Pleckstrin homology domain and a 'syntrophin unique' domain.

Beta-sarcoglycan is a protein that in humans is encoded by the SGCB gene.

Collagen alpha-3(VI) chain is a protein that in humans is encoded by the COL6A3 gene. This protein is an alpha chain of type VI collagen that aids in microfibril formation. As part of type VI collagen, this protein has been implicated in Bethlem myopathy, Ullrich congenital muscular dystrophy (UCMD), and other diseases related to muscle and connective tissue.

Delta-sarcoglycan is a protein that in humans is encoded by the SGCD gene.

Gamma-sarcoglycan is a protein that in humans is encoded by the SGCG gene. The α to δ-sarcoglycans are expressed predominantly (β) or exclusively in striated muscle. A mutation in any of the sarcoglycan genes may lead to a secondary deficiency of the other sarcoglycan proteins, presumably due to destabilisation of the sarcoglycan complex. The disease-causing mutations in the α to δ genes cause disruptions within the dystrophin-associated protein (DAP) complex in the muscle cell membrane. The transmembrane components of the DAP complex link the cytoskeleton to the extracellular matrix in adult muscle fibres, and are essential for the preservation of the integrity of the muscle cell membrane.

Dystrobrevin alpha is a protein that in humans is encoded by the DTNA gene.

Pikachurin, also known as AGRINL (AGRINL) and EGF-like, fibronectin type-III and laminin G-like domain-containing protein (EGFLAM), is a protein that in humans is encoded by the EGFLAM gene.

Sarcoglycan zeta also known as SGCZ is a protein which in humans is encoded by the SGCZ gene.
In molecular biology, exon skipping is a form of RNA splicing used to cause cells to “skip” over faulty or misaligned sections (exons) of genetic code, leading to a truncated but still functional protein despite the genetic mutation.


















