
A crystallographic defect is an interruption of the regular patterns of arrangement of atoms or molecules in crystalline solids. The positions and orientations of particles, which are repeating at fixed distances determined by the unit cell parameters in crystals, exhibit a periodic crystal structure, but this is usually imperfect. Several types of defects are often characterized: point defects, line defects, planar defects, bulk defects. Topological homotopy establishes a mathematical method of characterization.
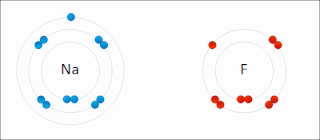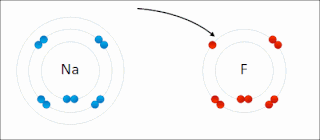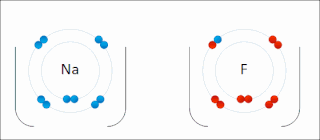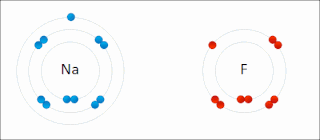
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds. It is one of the main types of bonding, along with covalent bonding and metallic bonding. Ions are atoms with an electrostatic charge. Atoms that gain electrons make negatively charged ions. Atoms that lose electrons make positively charged ions. This transfer of electrons is known as electrovalence in contrast to covalence. In the simplest case, the cation is a metal atom and the anion is a nonmetal atom, but these ions can be more complex, e.g. molecular ions like NH+
4 or SO2−
4. In simpler words, an ionic bond results from the transfer of electrons from a metal to a non-metal to obtain a full valence shell for both atoms.

In chemistry, a salt or ionic compound is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a neutral compound with no net electric charge. The constituent ions are held together by electrostatic forces termed ionic bonds.

An F center or Farbe center is a type of crystallographic defect in which an anionic vacancy in a crystal lattice is occupied by one or more unpaired electrons. Electrons in such a vacancy in a crystal lattice tend to absorb light in the visible spectrum such that a material that is usually transparent becomes colored. The greater the number of F centers, the more intense the color of the compound. F centers are a type of color center.
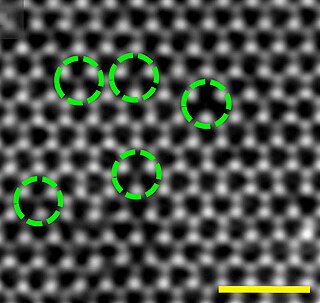
In crystallography, a vacancy is a type of point defect in a crystal where an atom is missing from one of the lattice sites. Crystals inherently possess imperfections, sometimes referred to as crystallographic defects.

Cerium(IV) oxide, also known as ceric oxide, ceric dioxide, ceria, cerium oxide or cerium dioxide, is an oxide of the rare-earth metal cerium. It is a pale yellow-white powder with the chemical formula CeO2. It is an important commercial product and an intermediate in the purification of the element from the ores. The distinctive property of this material is its reversible conversion to a non-stoichiometric oxide.

The Madelung constant is used in determining the electrostatic potential of a single ion in a crystal by approximating the ions by point charges. It is named after Erwin Madelung, a German physicist.
Ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the cation and anion gives the distance between the ions in a crystal lattice. Ionic radii are typically given in units of either picometers (pm) or angstroms (Å), with 1 Å = 100 pm. Typical values range from 31 pm (0.3 Å) to over 200 pm (2 Å).

Uranium dioxide or uranium(IV) oxide , also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reactors. A mixture of uranium and plutonium dioxides is used as MOX fuel. Prior to 1960, it was used as yellow and black color in ceramic glazes and glass.
In chemistry, the lattice energy is the energy change upon formation of one mole of a crystalline ionic compound from its constituent ions, which are assumed to initially be in the gaseous state. It is a measure of the cohesive forces that bind ionic solids. The size of the lattice energy is connected to many other physical properties including solubility, hardness, and volatility. Since it generally cannot be measured directly, the lattice energy is usually deduced from experimental data via the Born–Haber cycle.

Non-stoichiometric compounds are chemical compounds, almost always solid inorganic compounds, having elemental composition whose proportions cannot be represented by a ratio of small natural numbers ; most often, in such materials, some small percentage of atoms are missing or too many atoms are packed into an otherwise perfect lattice work.
In crystallography, a Frenkel defect is a type of point defect in crystalline solids, named after its discoverer Yakov Frenkel. The defect forms when an atom or smaller ion leaves its place in the structure, creating a vacancy and becomes an interstitial by lodging in a nearby location. In elemental systems, they are primarily generated during particle irradiation, as their formation enthalpy is typically much higher than for other point defects, such as vacancies, and thus their equilibrium concentration according to the Boltzmann distribution is below the detection limit. In ionic crystals, which usually possess low coordination number or a considerable disparity in the sizes of the ions, this defect can be generated also spontaneously, where the smaller ion is dislocated. Similar to a Schottky defect the Frenkel defect is a stoichiometric defect. In ionic compounds, the vacancy and interstitial defect involved are oppositely charged and one might expect them to be located close to each other due to electrostatic attraction. However, this is not likely the case in real material due to smaller entropy of such a coupled defect, or because the two defects might collapse into each other. Also, because such coupled complex defects are stoichiometric, their concentration will be independent of chemical conditions.
Kröger–Vink notation is a set of conventions that are used to describe electric charges and lattice positions of point defect species in crystals. It is primarily used for ionic crystals and is particularly useful for describing various defect reactions. It was proposed by F. A. Kröger and H. J. Vink.
In chemistry, a Zintl phase is a product of a reaction between a group 1 or group 2 and main group metal or metalloid. It is characterized by intermediate metallic/ionic bonding. Zintl phases are a subgroup of brittle, high-melting intermetallic compounds that are diamagnetic or exhibit temperature-independent paramagnetism and are poor conductors or semiconductors.

Solid-state ionics is the study of ionic-electronic mixed conductor and fully ionic conductors and their uses. Some materials that fall into this category include inorganic crystalline and polycrystalline solids, ceramics, glasses, polymers, and composites. Solid-state ionic devices, such as solid oxide fuel cells, can be much more reliable and long-lasting, especially under harsh conditions, than comparable devices with fluid electrolytes.
Transition metal oxides are compounds composed of oxygen atoms bound to transition metals. They are commonly utilized for their catalytic activity and semiconducting properties. Transition metal oxides are also frequently used as pigments in paints and plastics, most notably titanium dioxide. Transition metal oxides have a wide variety of surface structures which affect the surface energy of these compounds and influence their chemical properties. The relative acidity and basicity of the atoms present on the surface of metal oxides are also affected by the coordination of the metal cation and oxygen anion, which alter the catalytic properties of these compounds. For this reason, structural defects in transition metal oxides greatly influence their catalytic properties. The acidic and basic sites on the surface of metal oxides are commonly characterized via infrared spectroscopy, calorimetry among other techniques. Transition metal oxides can also undergo photo-assisted adsorption and desorption that alter their electrical conductivity. One of the more researched properties of these compounds is their response to electromagnetic radiation, which makes them useful catalysts for redox reactions, isotope exchange and specialized surfaces.
The strength of metal oxide adhesion effectively determines the wetting of the metal-oxide interface. The strength of this adhesion is important, for instance, in production of light bulbs and fiber-matrix composites that depend on the optimization of wetting to create metal-ceramic interfaces. The strength of adhesion also determines the extent of dispersion on catalytically active metal. Metal oxide adhesion is important for applications such as complementary metal oxide semiconductor devices. These devices make possible the high packing densities of modern integrated circuits.
Antiperovskites is a type of crystal structure similar to the perovskite structure that is common in nature. The key difference is that the positions of the cation and anion constituents are reversed in the unit cell structure. In contrast to perovskite, antiperovskite compounds consist of two types of anions coordinated with one type of cation. Antiperovskite compounds are an important class of materials because they exhibit interesting and useful physical properties not found in perovskite materials, including as electrolytes in solid-state batteries.

In crystallography, an anti-structure is obtained from a salt structure by exchanging anion and cation positions.
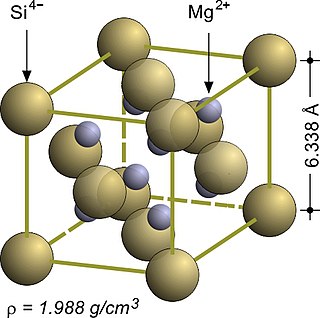
In solid state chemistry, the fluorite structure refers to a common motif for compounds with the formula MX2. The X ions occupy the eight tetrahedral interstitial sites whereas M ions occupy the regular sites of a face-centered cubic (FCC) structure. Many compounds, notably the common mineral fluorite (CaF2), adopt this structure.

















