Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. Autoxidation leads to degradation of organic compounds, including living matter. Antioxidants are frequently added to industrial products, such as polymers, fuels, and lubricants, to extend their usable lifetimes. Foods are also treated with antioxidants to forestall spoilage, in particular the rancidification of oils and fats. In cells, antioxidants such as glutathione, mycothiol or bacillithiol, and enzyme systems like superoxide dismutase, can prevent damage from oxidative stress.

Selenium is a chemical element; it has symbol Se and atomic number 34. It is a nonmetal with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It seldom occurs in its elemental state or as pure ore compounds in Earth's crust. Selenium was discovered in 1817 by Jöns Jacob Berzelius, who noted the similarity of the new element to the previously discovered tellurium.

Taurine, or 2-aminoethanesulfonic acid, is a non-proteinogenic amino sulfonic acid that is widely distributed in animal tissues. It is a major constituent of bile and can be found in the large intestine, and accounts for up to 0.1% of total human body weight.
A nutrient is a substance used by an organism to survive, grow, and reproduce. The requirement for dietary nutrient intake applies to animals, plants, fungi, and protists. Nutrients can be incorporated into cells for metabolic purposes or excreted by cells to create non-cellular structures, such as hair, scales, feathers, or exoskeletons. Some nutrients can be metabolically converted to smaller molecules in the process of releasing energy, such as for carbohydrates, lipids, proteins, and fermentation products, leading to end-products of water and carbon dioxide. All organisms require water. Essential nutrients for animals are the energy sources, some of the amino acids that are combined to create proteins, a subset of fatty acids, vitamins and certain minerals. Plants require more diverse minerals absorbed through roots, plus carbon dioxide and oxygen absorbed through leaves. Fungi live on dead or living organic matter and meet nutrient needs from their host.
In molecular biology a selenoprotein is any protein that includes a selenocysteine amino acid residue. Among functionally characterized selenoproteins are five glutathione peroxidases (GPX) and three thioredoxin reductases, (TrxR/TXNRD) which both contain only one Sec. Selenoprotein P is the most common selenoprotein found in the plasma. It is unusual because in humans it contains 10 Sec residues, which are split into two domains, a longer N-terminal domain that contains 1 Sec, and a shorter C-terminal domain that contains 9 Sec. The longer N-terminal domain is likely an enzymatic domain, and the shorter C-terminal domain is likely a means of safely transporting the very reactive selenium atom throughout the body.

Astaxanthin is a keto-carotenoid within a group of chemical compounds known as terpenes. Astaxanthin is a metabolite of zeaxanthin and canthaxanthin, containing both hydroxyl and ketone functional groups. It is a lipid-soluble pigment with red coloring properties, which result from the extended chain of conjugated double bonds at the center of the compound. The presence of the hydroxyl functional groups and the hydrophobic hydrocarbons render the molecule amphiphilic.

Canthaxanthin is a keto-carotenoid pigment widely distributed in nature. Carotenoids belong to a larger class of phytochemicals known as terpenoids. The chemical formula of canthaxanthin is C40H52O2. It was first isolated in edible mushrooms. It has also been found in green algae, bacteria, crustaceans, and bioaccumulates in fish such as carp, golden grey mullet, seabream and trush wrasse.

Selenomethionine (SeMet) is a naturally occurring amino acid. The L-selenomethionine enantiomer is the main form of selenium found in Brazil nuts, cereal grains, soybeans, and grassland legumes, while Se-methylselenocysteine, or its γ-glutamyl derivative, is the major form of selenium found in Astragalus, Allium, and Brassica species. In vivo, selenomethionine is randomly incorporated instead of methionine. Selenomethionine is readily oxidized.

Glucomannan is a water-soluble polysaccharide that is considered a dietary fiber. It is a hemicellulose component in the cell walls of some plant species. Glucomannan is a food additive used as an emulsifier and thickener. It is a major source of mannan oligosaccharide (MOS) found in nature, the other being galactomannan, which is insoluble.

Sodium selenite is the inorganic compound with the formula Na2SeO3. This salt is a colourless solid. The pentahydrate Na2SeO3(H2O)5 is the most common water-soluble selenium compound.

Selenium compounds are compounds containing the element selenium (Se). Among these compounds, selenium has various oxidation states, the most common ones being −2, +4, and +6. Selenium compounds exist in nature in the form of various minerals, such as clausthalite, guanajuatite, tiemannite, crookesite etc., and can also coexist with sulfide minerals such as pyrite and chalcopyrite. For many mammals, selenium compounds are essential. For example, selenomethionine and selenocysteine are selenium-containing amino acids present in the human body. Selenomethionine participates in the synthesis of selenoproteins. The reduction potential and pKa (5.47) of selenocysteine are lower than those of cysteine, making some proteins have antioxidant activity. Selenium compounds have important applications in semiconductors, glass and ceramic industries, medicine, metallurgy and other fields.
Natural growth promoters (NGPs) are feed additives for farm animals.
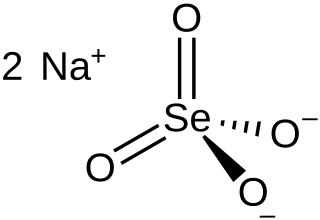
Sodium selenate is the inorganic compound with the formula Na
2SeO
4, not to be confused with sodium selenite. It exists as the anhydrous salt, the heptahydrate, and the decahydrate. These are white, water-soluble solids. The decahydrate is a common ingredient in multivitamins and livestock feed as a source of selenium. The anhydrous salt is used in the production of some glass. Although the selenates are much more toxic, many physical properties of sodium selenate and sodium sulfate are similar.

Animal feed is food given to domestic animals, especially livestock, in the course of animal husbandry. There are two basic types: fodder and forage. Used alone, the word feed more often refers to fodder. Animal feed is an important input to animal agriculture, and is frequently the main cost of the raising or keeping of animals. Farms typically try to reduce cost for this food, by growing their own, grazing animals, or supplementing expensive feeds with substitutes, such as food waste like spent grain from beer brewing.

Chelates in animal feed is jargon for metalloorganic compounds added to animal feed. The compounds provide sources of various metals that improve the health or marketability of the animal. Typical metals salts are derived from cobalt, copper, iron, manganese, and zinc. The objective of supplementation with trace minerals is to avoid a variety of deficiency diseases. Trace minerals carry out key functions in relation to many metabolic processes, most notably as cofactors for enzymes and hormones, and are essential for optimum health, growth and productivity. For example, supplementary minerals help ensure good growth, bone development, feathering in birds, hoof, skin and hair quality in mammals, enzyme structure and functions, and appetite. Deficiency of trace minerals affect many metabolic processes and so may be manifested by different symptoms, such as poor growth and appetite, reproductive failures, impaired immune responses, and general ill-thrift. From the 1950s to the 1990s most trace mineral supplementation of animal diets was in the form of inorganic minerals, and these largely eradicated associated deficiency diseases in farm animals. The role in fertility and reproductive diseases of dairy cattle highlights that organic forms of Zn are retained better than inorganic sources and so may provide greater benefit in disease prevention, notably mastitis and lameness.
Selenium deficiency occurs when an organism lacks the required levels of selenium, a critical nutrient in many species. Deficiency, although relatively rare in healthy well-nourished individuals, can have significant negative results, affecting the health of the heart and the nervous system; contributing to depression, anxiety, and dementia; and interfering with reproduction and gestation.

Selenium is an essential micronutrient for animals, though it is toxic in large doses. In plants, it sometimes occurs in toxic amounts as forage, e.g. locoweed. Selenium is a component of the amino acids selenocysteine and selenomethionine. In humans, selenium is a trace element nutrient that functions as cofactor for glutathione peroxidases and certain forms of thioredoxin reductase. Selenium-containing proteins are produced from inorganic selenium via the intermediacy of selenophosphate (PSeO33−).
Canadian Reference Materials (CRM) are certified reference materials of high-quality and reliability produced by the National Metrology Institute of Canada – the National Research Council Canada. The NRC Certified Reference Materials program is operated by the Measurement Science and Standards portfolio and provides CRMs for environmental, biotoxin, food, nutritional supplement, and stable isotope analysis. The program was established in 1976 to produce CRMs for inorganic and organic marine environmental analysis and remains internationally recognized producer of CRMs.

Methylselenocysteine, also known as Se-methylselenocysteine, is an analog of S-methylcysteine in which the sulfur atom is replaced with a selenium atom. It is an inhibitor of DMBA-induced mammary tumors and a "chemopreventive agent that blocks cell cycle progression and proliferation of premalignant mammary lesions and induces apoptosis of cancer cell lines in culture."

Arsonic acids are a subset of organoarsenic compounds defined as oxyacids where a pentavalent arsenic atom is bonded to two hydroxyl groups, a third oxygen atom, and an organic substituent. The salts/conjugate bases of arsonic acids are called arsonates. Like all arsenic-containing compounds, arsonic acids are toxic and carcinogenic to humans.














