Limestone is a common type of carbonate sedimentary rock. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of calcium carbonate. Limestone forms when these minerals precipitate out of water containing dissolved calcium. This can take place through both biological and nonbiological processes, though biological processes have likely been more important for the last 540 million years. Limestone often contains fossils, and these provide scientists with information on ancient environments and on the evolution of life.

Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO3). The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 3 as "calcite".

Calcium carbonate is a chemical compound with the formula CaCO3. It is a common substance found in rocks as the minerals calcite and aragonite (most notably as limestone, which is a type of sedimentary rock consisting mainly of calcite) and is the main component of eggshells, snail shells, seashells and pearls. Calcium carbonate is the active ingredient in agricultural lime and is created when calcium ions in hard water react with carbonate ions to create limescale. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues.

The lysocline is the depth in the ocean dependent upon the carbonate compensation depth (CCD), usually around 3.5 km, below which the rate of dissolution of calcite increases dramatically because of a pressure effect. While the lysocline is the upper bound of this transition zone of calcite saturation, the CCD is the lower bound of this zone.

Aragonite (IMA symbol: Arg) is a carbonate mineral, one of the three most common naturally occurring crystal forms of calcium carbonate, CaCO3 (the other forms being the minerals calcite and vaterite). It is formed by biological and physical processes, including precipitation from marine and freshwater environments.

Hard water is water that has high mineral content. Hard water is formed when water percolates through deposits of limestone, chalk or gypsum which are largely made up of calcium and magnesium carbonates, bicarbonates and sulfates.

Ooids are small, spheroidal, "coated" (layered) sedimentary grains, usually composed of calcium carbonate, but sometimes made up of iron- or phosphate-based minerals. Ooids usually form on the sea floor, most commonly in shallow tropical seas. After being buried under additional sediment, these ooid grains can be cemented together to form a sedimentary rock called an oolite. Oolites usually consist of calcium carbonate; these belong to the limestone rock family. Pisoids are similar to ooids, but are larger than 2 mm in diameter, often considerably larger, as with the pisoids in the hot springs at Carlsbad in the Czech Republic.

Dolomite (also known as dolomite rock, dolostone or dolomitic rock) is a sedimentary carbonate rock that contains a high percentage of the mineral dolomite, CaMg(CO3)2. It occurs widely, often in association with limestone and evaporites, though it is less abundant than limestone and rare in Cenozoic rock beds (beds less than about 65 million years in age). The first geologist to distinguish dolomite rock from limestone was Belsazar Hacquet in 1778.

Ocean acidification is the ongoing decrease in the pH value of the Earth's oceans, caused by the uptake of carbon dioxide (CO2) from the atmosphere. The main cause of ocean acidification is the burning of fossil fuels. Ocean acidification is one of several effects of climate change on oceans. Seawater is slightly basic (meaning pH > 7), and ocean acidification involves a shift towards pH-neutral conditions rather than a transition to acidic conditions (pH < 7). The concern with ocean acidification is that it can lead to the decreased production of the shells of shellfish and other aquatic life with calcium carbonate shells, as well as some other physiological challenges for marine organisms. The calcium carbonate shelled organisms can not reproduce under high saturated acidotic waters. An estimated 30–40% of the carbon dioxide from human activity released into the atmosphere dissolves into oceans, rivers and lakes. Some of it reacts with the water to form carbonic acid. Some of the resulting carbonic acid molecules dissociate into a bicarbonate ion and a hydrogen ion, thus increasing ocean acidity (H+ ion concentration).
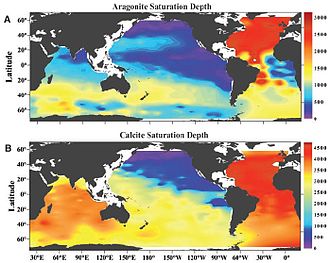
Carbonate compensation depth (CCD) is the depth in the oceans below which the rate of supply of calcite lags behind the rate of solvation, such that no calcite is preserved. Shells of animals therefore dissolve and carbonate particles may not accumulate in the sediments on the sea floor below this depth. Aragonite compensation depth describes the same behaviour in reference to aragonitic carbonates. Aragonite is more soluble than calcite, so the aragonite compensation depth is generally shallower than the calcite compensation depth.

A calcite sea is a sea in which low-magnesium calcite is the primary inorganic marine calcium carbonate precipitate. An aragonite sea is the alternate seawater chemistry in which aragonite and high-magnesium calcite are the primary inorganic carbonate precipitates. The Early Paleozoic and the Middle to Late Mesozoic oceans were predominantly calcite seas, whereas the Middle Paleozoic through the Early Mesozoic and the Cenozoic are characterized by aragonite seas ).

An aragonite sea contains aragonite and high-magnesium calcite as the primary inorganic calcium carbonate precipitates. The chemical conditions of the seawater must be notably high in magnesium content relative to calcium for an aragonite sea to form. This is in contrast to a calcite sea in which seawater low in magnesium content relative to calcium favors the formation of low-magnesium calcite as the primary inorganic marine calcium carbonate precipitate.
Ocean chemistry, also known as marine chemistry, is influenced by plate tectonics and seafloor spreading, turbidity currents, sediments, pH levels, atmospheric constituents, metamorphic activity, and ecology. The field of chemical oceanography studies the chemistry of marine environments including the influences of different variables. Marine life has adapted to the chemistries unique to earth's oceans, and marine ecosystems are sensitive to changes in ocean chemistry.

Shallow water marine environment refers to the area between the shore and deeper water, such as a reef wall or a shelf break. This environment is characterized by oceanic, geological and biological conditions, as described below. The water in this environment is shallow and clear, allowing the formation of different sedimentary structures, carbonate rocks, coral reefs, and allowing certain organisms to survive and become fossils.
Estuarine acidification happens when the pH balance of water in coastal marine ecosystems, specifically those of estuaries, decreases. Water, generally considered neutral on the pH scale, normally perfectly balanced between alkalinity and acidity. While ocean acidification occurs due to the ongoing decrease in the pH of the Earth's oceans, caused by the absorption of carbon dioxide (CO2) from the atmosphere, pH change in estuaries is more complicated than in the open ocean due to direct impacts from land run-off, human impact, and coastal current dynamics. In the ocean, wave and wind movement allows carbon dioxide (CO2) to mixes with water (H2O) forming carbonic acid (H2CO3). Through wave motion this chemical bond is mixed up, allowing for the further break of the bond, eventually becoming carbonate (CO3) which is basic and helps form shells for ocean creatures, and two hydron molecules. This creates the potential for acidic threat since hydron ions readily bond with any Lewis Structure to form an acidic bond. This is referred to as an oxidation-reduction reaction.

Ocean acidification threatens the Great Barrier Reef by reducing the viability and strength of coral reefs. The Great Barrier Reef, considered one of the seven natural wonders of the world and a biodiversity hotspot, is located in Australia. Similar to other coral reefs, it is experiencing degradation due to ocean acidification. Ocean acidification results from a rise in atmospheric carbon dioxide, which is taken up by the ocean. This process can increase sea surface temperature, decrease aragonite, and lower the pH of the ocean. The more we burn fossil fuels, the more the ocean absorbs CO₂, resulting in ocean acidification.

Marine biogenic calcification is the process by which marine organisms such as oysters and clams form calcium carbonate. Seawater is full of dissolved compounds, ions and nutrients that organisms can use for energy and, in the case of calcification, to build shells and outer structures. Calcifying organisms in the ocean include molluscs, foraminifera, coccolithophores, crustaceans, echinoderms such as sea urchins, and corals. The shells and skeletons produced from calcification have important functions for the physiology and ecology of the organisms that create them.

The calcium cycle is a transfer of calcium between dissolved and solid phases. There is a continuous supply of calcium ions into waterways from rocks, organisms, and soils. Calcium ions are consumed and removed from aqueous environments as they react to form insoluble structures such as calcium carbonate and calcium silicate, which can deposit to form sediments or the exoskeletons of organisms. Calcium ions can also be utilized biologically, as calcium is essential to biological functions such as the production of bones and teeth or cellular function. The calcium cycle is a common thread between terrestrial, marine, geological, and biological processes. Calcium moves through these different media as it cycles throughout the Earth. The marine calcium cycle is affected by changing atmospheric carbon dioxide due to ocean acidification.

The Arctic ocean covers an area of 14,056,000 squared kilometers, and supports a diverse and important socioeconomic food web of organisms, despite its average water temperature being 32 degrees Fahrenheit. Over the last three decades, the Arctic Ocean has experienced drastic changes due to climate change. One of the changes is in the acidity levels of the ocean, which have been consistently increasing at twice the rate of the Pacific and Atlantic oceans. Arctic Ocean acidification is a result of feedback from climate system mechanisms, and is having negative impacts on Arctic Ocean ecosystems and the organisms that live within them.

Particulate inorganic carbon (PIC) can be contrasted with dissolved inorganic carbon (DIC), the other form of inorganic carbon found in the ocean. These distinctions are important in chemical oceanography. Particulate inorganic carbon is sometimes called suspended inorganic carbon. In operational terms, it is defined as the inorganic carbon in particulate form that is too large to pass through the filter used to separate dissolved inorganic carbon.