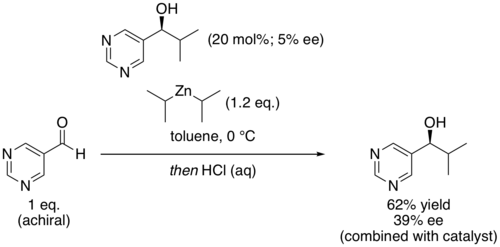
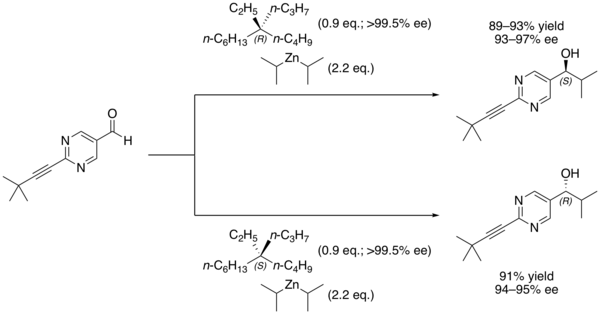
A chemical reaction is said to be autocatalytic if one of the reaction products is also a catalyst for the same reaction. Many forms of autocatalysis are recognized.

Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric products in unequal amounts."
1,1′-Bi-2-naphthol (BINOL) is an organic compound that is often used as a ligand for transition-metal catalysed asymmetric synthesis. BINOL has axial chirality and the two enantiomers can be readily separated and are stable toward racemisation. The specific rotation of the two enantiomers is 35.5° (c = 1 in THF), with the R enantiomer being the dextrorotary one. BINOL is a precursor for another chiral ligand called BINAP. The volumetric mass density of the two enantiomers is 0.62 g cm−3.
Homochirality is a uniformity of chirality, or handedness. Objects are chiral when they cannot be superposed on their mirror images. For example, the left and right hands of a human are approximately mirror images of each other but are not their own mirror images, so they are chiral. In biology, 19 of the 20 natural amino acids are homochiral, being L-chiral (left-handed), while sugars are D-chiral (right-handed). Homochirality can also refer to enantiopure substances in which all the constituents are the same enantiomer, but some sources discourage this use of the term.

In stereochemistry, a chiral auxiliary is a stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality present in the auxiliary can bias the stereoselectivity of one or more subsequent reactions. The auxiliary can then be typically recovered for future use.
In chemistry, transfer hydrogenation is a chemical reaction involving the addition of hydrogen to a compound from a source other than molecular H2. It is applied in laboratory and industrial organic synthesis to saturate organic compounds and reduce ketones to alcohols, and imines to amines. It avoids the need for high-pressure molecular H2 used in conventional hydrogenation. Transfer hydrogenation usually occurs at mild temperature and pressure conditions using organic or organometallic catalysts, many of which are chiral, allowing efficient asymmetric synthesis. It uses hydrogen donor compounds such as formic acid, isopropanol or dihydroanthracene, dehydrogenating them to CO2, acetone, or anthracene respectively. Often, the donor molecules also function as solvents for the reaction. A large scale application of transfer hydrogenation is coal liquefaction using "donor solvents" such as tetralin.

In stereochemistry, cryptochirality is a special case of chirality in which a molecule is chiral but its specific rotation is non-measurable. The underlying reason for the lack of rotation is the specific electronic properties of the molecule. The term was introduced by Kurt Mislow in 1977.
The Meerwein–Ponndorf–Verley (MPV) reduction in organic chemistry is the reduction of ketones and aldehydes to their corresponding alcohols utilizing aluminium alkoxide catalysis in the presence of a sacrificial alcohol. The advantages of the MPV reduction lie in its high chemoselectivity, and its use of a cheap environmentally friendly metal catalyst. MPV reductions have been described as "obsolete" owing to the development of sodium borohydride and related reagents.

In analytical chemistry, A chiral derivatizing agent (CDA), also known as a chiral resolving reagent, is a derivatization reagent that is a chiral auxiliary used to convert a mixture of enantiomers into diastereomers in order to analyze the quantities of each enantiomer present and determine the optical purity of a sample. Analysis can be conducted by spectroscopy or by chromatography. Some analytical techniques such as HPLC and NMR, in their most commons forms, cannot distinguish enantiomers within a sample, but can distinguish diastereomers. Therefore, converting a mixture of enantiomers to a corresponding mixture of diastereomers can allow analysis. The use of chiral derivatizing agents has declined with the popularization of chiral HPLC. Besides analysis, chiral derivatization is also used for chiral resolution, the actual physical separation of the enantiomers.

Diisopropylzinc is an organozinc compound with the chemical formula ZnC6H14.

In organic chemistry, organocatalysis is a form of catalysis in which the rate of a chemical reaction is increased by an organic catalyst. This "organocatalyst" consists of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds. Because of their similarity in composition and description, they are often mistaken as a misnomer for enzymes due to their comparable effects on reaction rates and forms of catalysis involved.
Asymmetric hydrogenation is a chemical reaction that adds two atoms of hydrogen to a target (substrate) molecule with three-dimensional spatial selectivity. Critically, this selectivity does not come from the target molecule itself, but from other reagents or catalysts present in the reaction. This allows spatial information to transfer from one molecule to the target, forming the product as a single enantiomer. The chiral information is most commonly contained in a catalyst and, in this case, the information in a single molecule of catalyst may be transferred to many substrate molecules, amplifying the amount of chiral information present. Similar processes occur in nature, where a chiral molecule like an enzyme can catalyse the introduction of a chiral centre to give a product as a single enantiomer, such as amino acids, that a cell needs to function. By imitating this process, chemists can generate many novel synthetic molecules that interact with biological systems in specific ways, leading to new pharmaceutical agents and agrochemicals. The importance of asymmetric hydrogenation in both academia and industry contributed to two of its pioneers — William Standish Knowles and Ryōji Noyori — being awarded one half of the 2001 Nobel Prize in Chemistry.
Spontaneous absolute asymmetric synthesis is a chemical phenomenon that stochastically generates chirality based on autocatalysis and small fluctuations in the ratio of enantiomers present in a racemic mixture. In certain reactions which initially do not contain chiral information, stochastically distributed enantiomeric excess can be observed. The phenomenon is different from chiral amplification, where enantiomeric excess is present from the beginning and not stochastically distributed. Hence, when the experiment is repeated many times, the average enantiomeric excess approaches 0%. The phenomenon has important implications concerning the origin of homochirality in nature.
In enantioselective synthesis, a non-linear effect refers to a process in which the enantiopurity of the catalyst or chiral auxiliary does not correspond with the enantiopurity of the product produced. For example: a racemic catalyst would be expected to convert a prochiral substrate into a racemic product, but this is not always the case and a chirally enriched product can be produced instead.
In organic chemistry, the Keck asymmetric allylation is a chemical reaction that involves the nucleophilic addition of an allyl group to an aldehyde. The catalyst is a chiral complex that contains titanium as a Lewis acid. The chirality of the catalyst induces a stereoselective addition, so the secondary alcohol of the product has a predictable absolute stereochemistry based on the choice of catalyst. This name reaction is named for Gary Keck.
The asymmetric addition of alkynylzinc compounds to aldehydes is an example of a Nef synthesis, a chemical reaction whereby a chiral propargyl alcohol is prepared from a terminal alkyne and an aldehyde. This alkynylation reaction is enantioselective and involves an alkynylzinc reagent rather than the sodium acetylide used by John Ulric Nef in his 1899 report of the synthetic approach. Propargyl alcohols are versatile precursors for the chirally-selective synthesis of natural products and pharmaceutical agents, making this asymmetric addition reaction of alkynylzinc compounds useful. For example, Erick Carreira used this approach in a total synthesis of the marine natural product leucascandrolide A, a bioactive metabolite of the calcareous sponge Leucascandra caveolata with cytotoxic and antifungal properties isolated in 1996.
Donna Blackmond is an American chemical engineer and the John C. Martin Endowed Chair in Chemistry at Scripps Research in La Jolla, CA. Her research focuses on prebiotic chemistry, the origin of biological homochirality, and kinetics and mechanisms of asymmetric catalytic reactions. Notable works include the development of Reaction Progress Kinetic Analysis (RPKA), analysis of non-linear effects of catalyst enantiopurity, biological homochirality and amino acid behavior.

The Krische allylation involves the enantioselective iridium-catalyzed addition of an allyl group to an aldehyde or an alcohol, resulting in the formation of a secondary homoallylic alcohol. The mechanism of the Krische allylation involves primary alcohol dehydrogenation or, when using aldehyde reactants, hydrogen transfer from 2-propanol. Unlike other allylation methods, the Krische allylation avoids the use of preformed allyl metal reagents and enables the direct conversion of primary alcohols to secondary homoallylic alcohols.
Viedma ripening or attrition-enhanced deracemization is a chiral symmetry breaking phenomenon observed in solid/liquid mixtures of enantiomorphous crystals that are subjected to comminution. It can be classified in the wider area of spontaneous symmetry breaking phenomena observed in chemistry and physics.
Kensō Soai is a Japanese organic chemist. He is a university lecturer in the Applied Chemistry Department of Tokyo University of Science.