In chemistry, a stepwise reaction [1] (also called an overall reaction, complex reaction, and multistep reaction, among others) is a chemical reaction with one or more reaction intermediates, which by definition involves at least two consecutive elementary reactions.
In a stepwise reaction, not all bonds are broken and formed at the same time. Hence, intermediates appear in the reaction pathway going from the reactants to the products. A stepwise reaction distinguishes itself from an elementary reaction in which the transformation is assumed to occur in a single step and to pass through a single transition state. [2]
In contrast to elementary reactions which follow the law of mass action, the rate law of stepwise reactions is obtained by combining the rate laws of the multiple elementary steps, and can become rather complex. Moreover, when speaking about catalytic reactions, the diffusion may also limit the reaction. In general, however, there is one very slow step, which is the rate-determining step, i.e. the reaction doesn't proceed any faster than the rate-determining step proceeds.
Organic reactions, especially when involving catalysis, are often stepwise. For example, a typical enol reaction consists of at least these elementary steps:
Rδ+ is an electron acceptor, for example, the carbon of a carbonyl (C=O). A very strong base, usually an alkoxide, is needed for the first step.
Reaction intermediates may be trapped in a trapping reaction. This proves the stepwise nature of the reaction and the structure of the intermediate. For example, superacids were used to prove the existence of carbocations.

A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei, and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur.
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.
In polymer chemistry, polymerization, or polymerisation, is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many forms of polymerization and different systems exist to categorize them.

An acetal is a functional group with the connectivity R2C(OR')2). Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments not hydrogen. The two R' groups can be equivalent to each other (a "symmetric acetal") or not (a "mixed acetal"). Acetals are formed from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon, but have substantially different chemical stability and reactivity as compared to the analogous carbonyl compounds. The central carbon atom has four bonds to it, and is therefore saturated and has tetrahedral geometry.
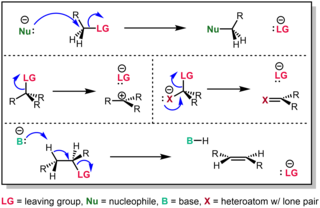
In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited to a fragment that departs with a pair of electrons in heterolytic bond cleavage. In this usage, a leaving group is a less formal but more commonly used synonym of the term nucleofuge. In this context, leaving groups are generally anions or neutral species, departing from a neutral or cationic substrates, respectively, though in rare cases, cations leaving from a dicationic substrate are also known. A species' ability to serve as a leaving group depends on its able to stabilize the additional electron density that results from bond heterolysis. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters such as tosylate (TsO−), while water (H2O), alcohols (HOR), and amines (R3N) are common neutral leaving groups.

The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit time. Reaction rates can vary dramatically. For example, the oxidative rusting of iron under Earth's atmosphere is a slow reaction that can take many years, but the combustion of cellulose in a fire is a reaction that takes place in fractions of a second. For most reactions, the rate decreases as the reaction proceeds. A reaction's rate can be determined by measuring the changes in concentration over time.
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is R−:C−R' or R=C: where the R represents substituents or hydrogen atoms.
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS) or rate-limiting step. For a given reaction mechanism, the prediction of the corresponding rate equation is often simplified by using this approximation of the rate-determining step.
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation. When their existence is indicated, reactive intermediates can help explain how a chemical reaction takes place.
The rate law or rate equation for a chemical reaction is an equation that links the initial or forward reaction rate with the concentrations or pressures of the reactants and constant parameters. For many reactions, the initial rate is given by a power law such as
Molecularity in chemistry is the number of molecules that come together to react in an elementary (single-step) reaction and is equal to the sum of stoichiometric coefficients of reactants in the elementary reaction with effective collision and correct orientation. Depending on how many molecules come together, a reaction can be unimolecular, bimolecular or even trimolecular.
The E1cB elimination reaction is a type of elimination reaction which occurs under basic conditions, where the hydrogen to be removed is relatively acidic, while the leaving group is a relatively poor one. Usually a moderate to strong base is present. E1cB is a two-step process, the first step of which may or may not be reversible. First, a base abstracts the relatively acidic proton to generate a stabilized anion. The lone pair of electrons on the anion then moves to the neighboring atom, thus expelling the leaving group and forming double or triple bond. The name of the mechanism - E1cB - stands for Elimination Unimolecular conjugate Base. Elimination refers to the fact that the mechanism is an elimination reaction and will lose two substituents. Unimolecular refers to the fact that the rate-determining step of this reaction only involves one molecular entity. Finally, conjugate base refers to the formation of the carbanion intermediate, which is the conjugate base of the starting material.
Chain propagation (sometimes referred to as propagation) is a process in which a reactive intermediate is continuously regenerated during the course of a chemical chain reaction. For example, in the chlorination of methane, there is a two-step propagation cycle involving as chain carriers a chlorine atom and a methyl radical which are regenerated alternately:
Chain termination is any chemical reaction that ceases the formation of reactive intermediates in a chain propagation step in the course of a polymerization, effectively bringing it to a halt.

Hammond's postulate, is a hypothesis in physical organic chemistry which describes the geometric structure of the transition state in an organic chemical reaction. First proposed by George Hammond in 1955, the postulate states that:
If two states, as, for example, a transition state and an unstable intermediate, occur consecutively during a reaction process and have nearly the same energy content, their interconversion will involve only a small reorganization of the molecular structures.
In chemistry, a reaction intermediate or an intermediate is a molecular entity that is formed from the reactants and reacts further to give the directly observed products of a chemical reaction. Most chemical reactions are stepwise, that is they take more than one elementary step to complete. An intermediate is the reaction product of each of these steps, except for the last one, which forms the final product.
An elementary reaction is a chemical reaction in which one or more chemical species react directly to form products in a single reaction step and with a single transition state. In practice, a reaction is assumed to be elementary if no reaction intermediates have been detected or need to be postulated to describe the reaction on a molecular scale. An apparently elementary reaction may be in fact a stepwise reaction, i.e. a complicated sequence of chemical reactions, with reaction intermediates of variable lifetimes.
A reaction step of a chemical reaction is defined as: "An elementary reaction, constituting one of the stages of a stepwise reaction in which a reaction intermediate is converted into the next reaction intermediate in the sequence of intermediates between reactants and products". To put it simply, it is an elementary reaction which goes from one reaction intermediate to another or to the final product.
This glossary of chemistry terms is a list of terms and definitions relevant to chemistry, including chemical laws, diagrams and formulae, laboratory tools, glassware, and equipment. Chemistry is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions; it features an extensive vocabulary and a significant amount of jargon.