
In organic chemistry, a diene ; also diolefin, dy-OH-lə-fin) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alkene units, with the standard prefix di of systematic nomenclature. As a subunit of more complex molecules, dienes occur in naturally occurring and synthetic chemicals and are used in organic synthesis. Conjugated dienes are widely used as monomers in the polymer industry. Polyunsaturated fats are of interest to nutrition.
Cubane is a synthetic hydrocarbon compound with the formula C8H8, and that consists of eight carbon atoms arranged at the corners of a cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons and a member of the prismanes. It was first synthesized in 1964 by Philip Eaton and Thomas Cole. Before this work, Eaton believed that cubane would be impossible to synthesize due to the "required 90 degree bond angles". The cubic shape requires the carbon atoms to adopt an unusually sharp 90° bonding angle, which would be highly strained as compared to the 109.45° angle of a tetrahedral carbon. Once formed, cubane is quite kinetically stable, due to a lack of readily available decomposition paths. It is the simplest hydrocarbon with octahedral symmetry.

Phosphorine is a heavier element analog of pyridine, containing a phosphorus atom instead of an aza- moiety. It is also called phosphabenzene and belongs to the phosphaalkene class. It is a colorless liquid that is mainly of interest in research.

A silabenzene is a heteroaromatic compound containing one or more silicon atoms instead of carbon atoms in benzene. A single substitution gives silabenzene proper; additional substitutions give a disilabenzene, trisilabenzene, etc.
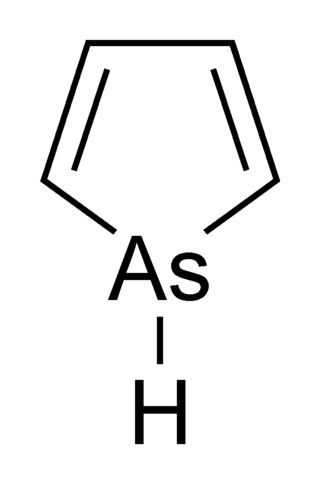
Arsole, also called arsenole or arsacyclopentadiene, is an organoarsenic compound with the formula C4H4AsH. It is classified as a metallole and is isoelectronic to and related to pyrrole except that an arsenic atom is substituted for the nitrogen atom. Whereas the pyrrole molecule is planar, the arsole molecule is not, and the hydrogen atom bonded to arsenic extends out of the molecular plane. Arsole is only moderately aromatic, with about 40% the aromaticity of pyrrole. Arsole itself has not been reported in pure form, but several substituted analogs called arsoles exist. Arsoles and more complex arsole derivatives have similar structure and chemical properties to those of phosphole derivatives. When arsole is fused to a benzene ring, this molecule is called arsindole, or benzarsole.
In organic chemistry, Madelung synthesis is a chemical reaction that produces indoles by the intramolecular cyclization of N-phenylamides using strong base at high temperature. The Madelung synthesis was reported in 1912 by Walter Madelung, when he observed that 2-phenylindole was synthesized using N-benzoyl-o-toluidine and two equivalents of sodium ethoxide in a heated, airless reaction. Common reaction conditions include use of sodium or potassium alkoxide as base in hexane or tetrahydrofuran solvents, at temperatures ranging between 200–400 °C. A hydrolysis step is also required in the synthesis. The Madelung synthesis is important because it is one of few known reactions that produce indoles from a base-catalyzed thermal cyclization of N-acyl-o-toluidines.
Squaric acid, also called quadratic acid because its four carbon atoms approximately form a square, is a diprotic organic acid with the chemical formula C4O2(OH)2.
In organic chemistry, umpolung or polarity inversion is the chemical modification of a functional group with the aim of the reversal of polarity of that group. This modification allows secondary reactions of this functional group that would otherwise not be possible. The concept was introduced by D. Seebach and E.J. Corey. Polarity analysis during retrosynthetic analysis tells a chemist when umpolung tactics are required to synthesize a target molecule.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.

Germabenzene (C5H6Ge) is the parent representative of a group of chemical compounds containing in their molecular structure a benzene ring with a carbon atom replaced by a germanium atom. Germabenzene itself has been studied theoretically, and synthesized with a bulky 2,4,6-tris[bis(trimethylsilyl)methyl]phenyl or Tbt group. Also, stable naphthalene derivatives do exist in the laboratory such as the 2-germanaphthalene-containing substance represented below. The germanium to carbon bond in this compound is shielded from potential reactants by a Tbt group. This compound is aromatic just as the other carbon group representatives silabenzene and stannabenzene.

Dewar benzene (also spelled dewarbenzene) or bicyclo[2.2.0]hexa-2,5-diene is a bicyclic isomer of benzene with the molecular formula C6H6. The compound is named after James Dewar who included this structure in a list of possible C6H6 structures in 1869. However, he did not propose it as the structure of benzene, and in fact he supported the correct structure previously proposed by August Kekulé in 1865.

Sulfolene, or butadiene sulfone is a cyclic organic chemical with a sulfone functional group. It is a white, odorless, crystalline, indefinitely storable solid, which dissolves in water and many organic solvents. The compound is used as a source of butadiene.

NanoPutians are a series of organic molecules whose structural formulae resemble human forms. James Tour's research group designed and synthesized these compounds in 2003 as a part of a sequence on chemical education for young students. The compounds consist of two benzene rings connected via a few carbon atoms as the body, four acetylene units each carrying an alkyl group at their ends which represents the hands and legs, and a 1,3-dioxolane ring as the head. Tour and his team at Rice University used the NanoPutians in their NanoKids educational outreach program. The goal of this program was to educate children in the sciences in an effective and enjoyable manner. They have made several videos featuring the NanoPutians as anthropomorphic animated characters.

Basketane is a polycyclic alkane with the chemical formula C10H12. The name is taken from its structural similarity to a basket shape. Basketane was first synthesized in 1966, independently by Masamune and Dauben and Whalen. A patent application published in 1988 used basketane, which is a hydrocarbon, as a source material in doping thin diamond layers because of the molecule's high vapor pressure, carbon ring structure, and fewer hydrogen-to-carbon bond ratio.
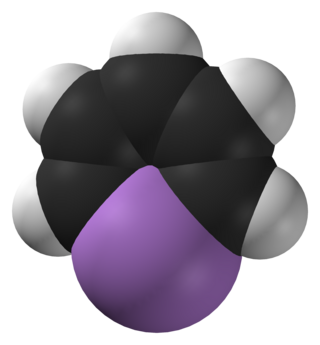
Arsabenzene (IUPAC name: arsinine) is an organoarsenic heterocyclic compound with the chemical formula C5H5As. It belongs to a group of compounds called heteroarenes that have the general formula C5H5E (E= N, P, As, Sb, Bi).

Bismole is a theoretical heterocyclic organic compound, a five-membered ring with the formula C4H4BiH. It is classified as a metallole. It can be viewed as a structural analog of pyrrole, with bismuth replacing the nitrogen atom of pyrrole. The unsubstituted compound has not been isolated due to the high energy of the Bi-H bond. Substituted derivatives, which have been synthesized, are called bismoles.

Bicyclopropenyl (bicycloprop-2-enyl, C6H6) is an organic compound and one of several valence isomers of benzene. The molecule can be described as two coupled cyclopropene units. The positions of the alkene groups can vary and therefore two other isomers are known: bicycloprop-1,2-enyl and bicyclopropen-1-yl.

Bismabenzene is the parent representative of a group of organobismuth compounds that are related to benzene with a carbon atom replaced by a bismuth atom. Bismabenzene itself has been synthesised but not isolated because it is too reactive, tending to instead dimerize in a Diels-Alder addition.

Selenopyrylium is an aromatic heterocyclic compound consisting of a six-membered ring with five carbon atoms and a positively charged selenium atom.

Boraacenes are polycyclic aromatic hydrocarbons containing at least one boron atom. Structurally, they are related to acenes, linearly fused benzene rings. However, the boron atom is electron deficient and may act as a Lewis Acid when compared to carbon. This results in slightly less negative charge within the ring, smaller HOMO-LUMO gaps, as well as differences in redox chemistry when compared to their acene analogues. When incorporated into acenes, Boron maintains the planarity and aromaticity of carbon acenes, while adding an empty p-orbital, which can be utilized for the fine tuning of organic semiconductor band gaps. Due to this empty p orbital, however, it is also highly reactive when exposed to nucleophiles like water or normal atmosphere, as it will readily be attacked by oxygen, which must be addressed to maintain its stability.






















