
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high-quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.

Niobium is a chemical element; it has symbol Nb and atomic number 41. It is a light grey, crystalline, and ductile transition metal. Pure niobium has a Mohs hardness rating similar to pure titanium, and it has similar ductility to iron. Niobium oxidizes in Earth's atmosphere very slowly, hence its application in jewelry as a hypoallergenic alternative to nickel. Niobium is often found in the minerals pyrochlore and columbite, hence the former name "columbium". Its name comes from Greek mythology: Niobe, daughter of Tantalus, the namesake of tantalum. The name reflects the great similarity between the two elements in their physical and chemical properties, which makes them difficult to distinguish.

Tantalum is a chemical element; it has symbol Ta and atomic number 73. Previously known as tantalium, it is named after Tantalus, a figure in Greek mythology. Tantalum is a very hard, ductile, lustrous, blue-gray transition metal that is highly corrosion-resistant. It is part of the refractory metals group, which are widely used as components of strong high-melting-point alloys. It is a group 5 element, along with vanadium and niobium, and it always occurs in geologic sources together with the chemically similar niobium, mainly in the mineral groups tantalite, columbite and coltan.
A thin film is a layer of material ranging from fractions of a nanometer (monolayer) to several micrometers in thickness. The controlled synthesis of materials as thin films is a fundamental step in many applications. A familiar example is the household mirror, which typically has a thin metal coating on the back of a sheet of glass to form a reflective interface. The process of silvering was once commonly used to produce mirrors, while more recently the metal layer is deposited using techniques such as sputtering. Advances in thin film deposition techniques during the 20th century have enabled a wide range of technological breakthroughs in areas such as magnetic recording media, electronic semiconductor devices, integrated passive devices, LEDs, optical coatings, hard coatings on cutting tools, and for both energy generation and storage. It is also being applied to pharmaceuticals, via thin-film drug delivery. A stack of thin films is called a multilayer.
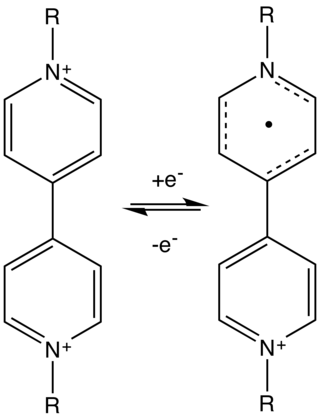
Electrochromism is a phenomenon in which a material displays changes in color or opacity in response to an electrical stimulus. In this way, a smart window made of an electrochromic material can block specific wavelengths of ultraviolet, visible or (near) infrared light. The ability to control the transmittance of near-infrared light can increase the energy efficiency of a building, reducing the amount of energy needed to cool during summer and heat during winter.
In materials science, the sol–gel process is a method for producing solid materials from small molecules. The method is used for the fabrication of metal oxides, especially the oxides of silicon (Si) and titanium (Ti). The process involves conversion of monomers into a colloidal solution (sol) that acts as the precursor for an integrated network of either discrete particles or network polymers. Typical precursors are metal alkoxides. Sol–gel process is used to produce ceramic nanoparticles.

Tantalum(V) chloride, also known as tantalum pentachloride, is an inorganic compound with the formula TaCl5. It takes the form of a white powder and is commonly used as a starting material in tantalum chemistry. It readily hydrolyzes to form tantalum(V) oxychloride (TaOCl3) and eventually tantalum pentoxide (Ta2O5); this requires that it be synthesised and manipulated under anhydrous conditions, using air-free techniques.

Tantalum pentoxide, also known as tantalum(V) oxide, is the inorganic compound with the formula Ta
2O
5. It is a white solid that is insoluble in all solvents but is attacked by strong bases and hydrofluoric acid. Ta
2O
5 is an inert material with a high refractive index and low absorption, which makes it useful for coatings. It is also extensively used in the production of capacitors, due to its high dielectric constant.
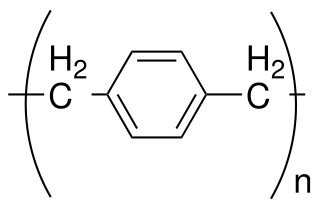
Parylene is the common name of a polymer whose backbone consists of para-benzenediyl rings −C
6H
4− connected by 1,2-ethanediyl bridges −CH
2−CH
2−. It can be obtained by polymerization of para-xylyleneH
2C=C
6H
4=CH
2.
An electrochromic device (ECD) controls optical properties such as optical transmission, absorption, reflectance and/or emittance in a continual but reversible manner on application of voltage (electrochromism). This property enables an ECD to be used for applications like smart glass, electrochromic mirrors, and electrochromic display devices.

Titanium isopropoxide, also commonly referred to as titanium tetraisopropoxide or TTIP, is a chemical compound with the formula Ti{OCH(CH3)2}4. This alkoxide of titanium(IV) is used in organic synthesis and materials science. It is a diamagnetic tetrahedral molecule. Titanium isopropoxide is a component of the Sharpless epoxidation, a method for the synthesis of chiral epoxides.
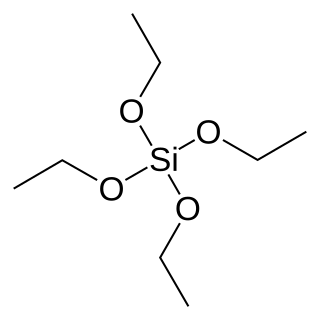
Tetraethyl orthosilicate, formally named tetraethoxysilane (TEOS), ethyl silicate is the organic chemical compound with the formula Si(OC2H5)4. TEOS is a colorless liquid. It degrades in water. TEOS is the ethyl ester of orthosilicic acid, Si(OH)4. It is the most prevalent alkoxide of silicon.

Niobium pentoxide is the inorganic compound with the formula Nb2O5. A colorless, insoluble, and fairly unreactive solid, it is the most widespread precursor for other compounds and materials containing niobium. It is predominantly used in alloying, with other specialized applications in capacitors, optical glasses, and the production of lithium niobate.

Tantalum borides are compounds of tantalum and boron most remarkable for their extreme hardness.
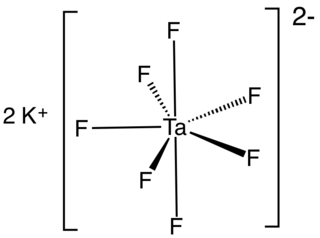
A tantalate is a tantalum-containing anion or a salt of such an anion. A commercially important example is heptafluorotantalate (TaF72−) and its potassium salt (K2TaF7).
Combustion chemical vapor deposition (CCVD) is a chemical process by which thin-film coatings are deposited onto substrates in the open atmosphere.
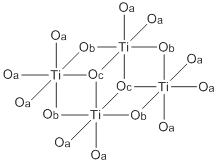
Titanium ethoxide is a chemical compound with the formula Ti4(OCH2CH3)16. It is a commercially available colorless liquid that is soluble in organic solvents but hydrolyzes readily. Its structure is more complex than suggested by its empirical formula. Like other alkoxides of titanium(IV) and zirconium(IV), it finds used in organic synthesis and materials science.

Niobium(V) ethoxide is an metalorganic compound with formula Nb2(OC2H5)10. It is a colorless liquid that dissolves in some organic solvents but hydrolyzes readily. It is mainly used for the sol-gel processing of materials containing niobium oxides.

Titanium butoxide is a metal alkoxide with the formula Ti(OBu)4 (Bu = –CH2CH2CH2CH3). It is a colorless odorless liquid although aged samples can appear yellowish. Owing to hydrolysis, samples have a weak alcohol-like odor. It is soluble in many organic solvents. Decomposition in water is not hazardous, and therefore titanium butoxide is often used as a liquid source of titanium dioxide, which allows deposition of TiO2 coatings of various shapes and sizes down to the nanoscale.

Niobium diselenide or niobium(IV) selenide is a layered transition metal dichalcogenide with formula NbSe2. Niobium diselenide is a lubricant, and a superconductor at temperatures below 7.2 K that exhibit a charge density wave (CDW). NbSe2 crystallizes in several related forms, and can be mechanically exfoliated into monatomic layers, similar to other transition metal dichalcogenide monolayers. Monolayer NbSe2 exhibits very different properties from the bulk material, such as of Ising superconductivity, quantum metallic state, and strong enhancement of the CDW.





















