Acetylcholine (ACh) is an organic compound that functions in the brain and body of many types of animals as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Parts in the body that use or are affected by acetylcholine are referred to as cholinergic.
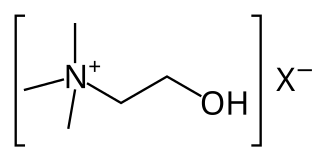
Cholinergic agents are compounds which mimic the action of acetylcholine and/or butyrylcholine. In general, the word "choline" describes the various quaternary ammonium salts containing the N,N,N-trimethylethanolammonium cation. Found in most animal tissues, choline is a primary component of the neurotransmitter acetylcholine and functions with inositol as a basic constituent of lecithin. Choline also prevents fat deposits in the liver and facilitates the movement of fats into cells.

The enzyme cholinesterase (EC 3.1.1.8, choline esterase; systematic name acylcholine acylhydrolase) catalyses the hydrolysis of choline-based esters:
Neurotoxicity is a form of toxicity in which a biological, chemical, or physical agent produces an adverse effect on the structure or function of the central and/or peripheral nervous system. It occurs when exposure to a substance – specifically, a neurotoxin or neurotoxicant– alters the normal activity of the nervous system in such a way as to cause permanent or reversible damage to nervous tissue. This can eventually disrupt or even kill neurons, which are cells that transmit and process signals in the brain and other parts of the nervous system. Neurotoxicity can result from organ transplants, radiation treatment, certain drug therapies, recreational drug use, exposure to heavy metals, bites from certain species of venomous snakes, pesticides, certain industrial cleaning solvents, fuels and certain naturally occurring substances. Symptoms may appear immediately after exposure or be delayed. They may include limb weakness or numbness, loss of memory, vision, and/or intellect, uncontrollable obsessive and/or compulsive behaviors, delusions, headache, cognitive and behavioral problems and sexual dysfunction. Chronic mold exposure in homes can lead to neurotoxicity which may not appear for months to years of exposure. All symptoms listed above are consistent with mold mycotoxin accumulation.

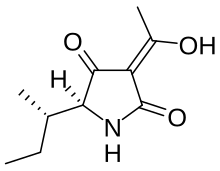
Physostigmine is a highly toxic parasympathomimetic alkaloid, specifically, a reversible cholinesterase inhibitor. It occurs naturally in the Calabar bean and the fruit of the Manchineel tree.

Plastoquinone (PQ) is a terpenoid-quinone (meroterpenoid) molecule involved in the electron transport chain in the light-dependent reactions of photosynthesis. The most common form of plastoquinone, known as PQ-A or PQ-9, is a 2,3-dimethyl-1,4-benzoquinone molecule with a side chain of nine isoprenyl units. There are other forms of plastoquinone, such as ones with shorter side chains like PQ-3 as well as analogs such as PQ-B, PQ-C, and PQ-D, which differ in their side chains. The benzoquinone and isoprenyl units are both nonpolar, anchoring the molecule within the inner section of a lipid bilayer, where the hydrophobic tails are usually found.
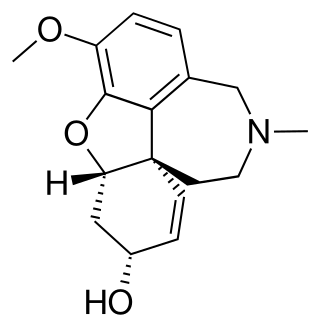
Galantamine is used for the treatment of cognitive decline in mild to moderate Alzheimer's disease and various other memory impairments. It is an alkaloid extracted from the bulbs and flowers of Galanthus nivalis, Galanthus caucasicus, Galanthus woronowii, and other members of the family Amaryllidaceae, such as Narcissus (daffodil), Leucojum aestivum (snowflake), and Lycoris including Lycoris radiata. It can also be produced synthetically.
Phytotoxins are substances that are poisonous or toxic to the growth of plants. Phytotoxic substances may result from human activity, as with herbicides, or they may be produced by plants, by microorganisms, or by naturally occurring chemical reactions.

Photoinhibition is light-induced reduction in the photosynthetic capacity of a plant, alga, or cyanobacterium. Photosystem II (PSII) is more sensitive to light than the rest of the photosynthetic machinery, and most researchers define the term as light-induced damage to PSII. In living organisms, photoinhibited PSII centres are continuously repaired via degradation and synthesis of the D1 protein of the photosynthetic reaction center of PSII. Photoinhibition is also used in a wider sense, as dynamic photoinhibition, to describe all reactions that decrease the efficiency of photosynthesis when plants are exposed to light.

Pheophytin or phaeophytin is a chemical compound that serves as the first electron carrier intermediate in the electron transfer pathway of Photosystem II in plants, and the type II photosynthetic reaction center found in purple bacteria. In both PS II and RC P870, light drives electrons from the reaction center through pheophytin, which then passes the electrons to a quinone (QA) in RC P870 and RC P680. The overall mechanisms, roles, and purposes of the pheophytin molecules in the two transport chains are analogous to each other.

Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters:

Photosynthetic reaction centre proteins are main protein components of photosynthetic reaction centres (RCs) of bacteria and plants. They are transmembrane proteins embedded in the chloroplast thylakoid or bacterial cell membrane.
Cytochrome b559 is an important component of Photosystem II (PSII) is a multisubunit protein-pigment complex containing polypeptides both intrinsic and extrinsic to the photosynthetic membrane. Within the core of the complex, the chlorophyll and beta-carotene pigments are mainly bound to the antenna proteins CP43 (PsbC) and CP47 (PsbB), which pass the excitation energy on to chlorophylls in the reaction centre proteins D1 and D2 that bind all the redox-active cofactors involved in the energy conversion process. The PSII oxygen-evolving complex (OEC) provides electrons to re-reduce the PSII reaction center, and oxidizes 2 water molecules to recover its reduced initial state. It consists of OEE1 (PsbO), OEE2 (PsbP) and OEE3 (PsbQ). The remaining subunits in PSII are of low molecular weight, and are involved in PSII assembly, stabilisation, dimerization, and photoprotection.

Acetylcholinesterase inhibitors (AChEIs) also often called cholinesterase inhibitors, inhibit the enzyme acetylcholinesterase from breaking down the neurotransmitter acetylcholine into choline and acetate, thereby increasing both the level and duration of action of acetylcholine in the central nervous system, autonomic ganglia and neuromuscular junctions, which are rich in acetylcholine receptors. Acetylcholinesterase inhibitors are one of two types of cholinesterase inhibitors; the other being butyryl-cholinesterase inhibitors. Acetylcholinesterase is the primary member of the cholinesterase enzyme family.

Rivastigmine is a cholinesterase inhibitor used for the treatment of mild to moderate Alzheimer's disease. The drug can be administered orally or via a transdermal patch; the latter form reduces the prevalence of side effects, which typically include nausea and vomiting.

Cymserine is a drug related to physostigmine, which acts as a reversible cholinesterase inhibitor, with moderate selectivity (15×) for the plasma cholinesterase enzyme butyrylcholinesterase, and relatively weaker inhibition of the better-known acetylcholinesterase enzyme. This gives it a much more specific profile of effects that may be useful for treating Alzheimer's disease without producing side effects such as tremors, lacrimation, and salivation that are seen with the older nonselective cholinesterase inhibitors currently used for this application, such as donepezil. A number of cymserine derivatives have been developed with much greater selectivity for butyrylcholinesterase, and both cymserine and several of its analogues have been tested in animals, and found to increase brain acetylcholine levels and produce nootropic effects, as well as reducing levels of amyloid precursor protein and amyloid beta, which are commonly used biomarkers for the development of Alzheimer's disease.

In molecular biology, the PsbZ (Ycf9) is a protein domain, which is low in molecular weight. It is a transmembrane protein and therefore is located in the thylakoid membrane of chloroplasts in cyanobacteria and plants. More specifically, it is located in Photosystem II (PSII) and in the light-harvesting complex II (LHCII). Ycf9 acts as a structural linker, that stabilises the PSII-LHCII supercomplexes. Moreover, the supercomplex fails to form in PsbZ-deficient mutants, providing further evidence to suggest Ycf9's role as a structural linker. This may be caused by a marked decrease in two LHCII antenna proteins, CP26 and CP29, found in PsbZ-deficient mutants, which result in structural changes, as well as functional modifications in PSII.
Methanesulfonyl fluoride (MSF) has long been known to be a potent inhibitor of acetylcholinesterase (AChE), the enzyme that regulates acetylcholine, an important neurotransmitter in both the central and peripheral nervous systems.

Phenserine is a synthetic drug which has been investigated as a medication to treat Alzheimer's disease (AD), as the drug exhibits neuroprotective and neurotrophic effects.
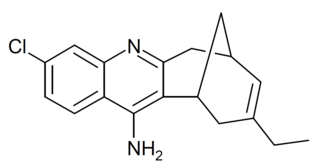
Huprine X is a synthetic cholinergic compound developed as a hybrid between the natural product Huperzine A and the synthetic drug tacrine. It is one of the most potent reversible inhibitors of acetylcholinesterase known, with a binding affinity of 0.026nM, as well as showing direct agonist activity at both nicotinic and muscarinic acetylcholine receptors. In animal studies it has nootropic and neuroprotective effects, and is used in research into Alzheimer's disease, and although huprine X itself has not been researched for medical use in humans, a large family of related derivatives have been developed.