A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles.
Garlic is a species of bulbous flowering plant in the genus Allium. Its close relatives include the onion, shallot, leek, chive, Welsh onion, and Chinese onion. It is native to South Asia, Central Asia and northeastern Iran and has long been used as a seasoning worldwide, with a history of several thousand years of human consumption and use. It was known to ancient Egyptians and has been used as both a food flavoring and a traditional medicine. China produced 73% of the world's supply of garlic in 2021.

In organic chemistry, a sulfide or thioether is an organosulfur functional group with the connectivity R−S−R' as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sulfide is similar to an ether except that it contains a sulfur atom in place of the oxygen. The grouping of oxygen and sulfur in the periodic table suggests that the chemical properties of ethers and sulfides are somewhat similar, though the extent to which this is true in practice varies depending on the application.

Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent.
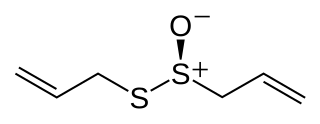
Allicin is an organosulfur compound obtained from garlic. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic. Allicin is unstable and quickly changes into a series of other sulfur-containing compounds such as diallyl disulfide. Allicin is an antifeedant, i.e. the defense mechanism against attacks by pests on the garlic plant.
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O), selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring.

Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. The parent PT is an insoluble colored solid with the formula (C4H2S)n. The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s have alkyl substituents at the 3- or 4-position(s). They are also colored solids, but tend to be soluble in organic solvents.

In organic chemistry, an allyl group is a substituent with the structural formula −CH2−HC=CH2. It consists of a methylene bridge attached to a vinyl group. The name is derived from the scientific name for garlic, Allium sativum. In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "Schwefelallyl". The term allyl applies to many compounds related to H2C=CH−CH2, some of which are of practical or of everyday importance, for example, allyl chloride.

Ajoene is an organosulfur compound found in garlic (Allium sativum) extracts. It is a colorless liquid that contains sulfoxide and disulfide functional groups. The name (and pronunciation) is derived from "ajo", the Spanish word for garlic. It is found as a mixture of up to four stereoisomers, which differ in terms of the stereochemistry of the central alkene (E- vs Z-) and the chirality of the sulfoxide sulfur (R- vs S-).

Ethionine is a non-proteinogenic amino acid structurally related to methionine, with an ethyl group in place of the methyl group.

Benzothiophene is an aromatic organic compound with a molecular formula C8H6S and an odor similar to naphthalene (mothballs). It occurs naturally as a constituent of petroleum-related deposits such as lignite tar. Benzothiophene has no household use. In addition to benzo[b]thiophene, a second isomer is known: benzo[c]thiophene.
The terpinenes are a group of isomeric hydrocarbons that are classified as monoterpenes. They each have the same molecular formula and carbon framework, but they differ in the position of carbon-carbon double bonds. α-Terpinene has been isolated from cardamom and marjoram oils, and from other natural sources. β-Terpinene has no known natural source but has been prepared from sabinene. γ-Terpinene and δ-terpinene have been isolated from a variety of plant sources. They are all colorless liquids with a turpentine-like odor.
In organic chemistry, the Paal–Knorr synthesis is a reaction used to synthesize substituted furans, pyrroles, or thiophenes from 1,4-diketones. It is a synthetically valuable method for obtaining substituted furans and pyrroles, which are common structural components of many natural products. It was initially reported independently by German chemists Carl Paal and Ludwig Knorr in 1884 as a method for the preparation of furans, and has been adapted for pyrroles and thiophenes. Although the Paal–Knorr synthesis has seen widespread use, the mechanism wasn't fully understood until it was elucidated by V. Amarnath et al. in the 1990s.

Sulfolene, or butadiene sulfone is a cyclic organic chemical with a sulfone functional group. It is a white, odorless, crystalline, indefinitely storable solid, which dissolves in water and many organic solvents. The compound is used as a source of butadiene.

In enzymology, an alliin lyase is an enzyme that catalyzes the chemical reaction
Chester J. Cavallito was an American organic chemist. He was particularly known for his work on the chemistry of garlic. Beginning in 1944, with his colleagues, he reported on the isolation from crushed garlic, synthesis and antibiotic activity of a compound he named allicin. Cavallito established that allicin was a member of a class of organosulfur compounds known as thiosulfinates. He also synthesized and reported on the chemical and biological properties of a series of thiosulfinates related to allicin.

Allium is a genus of monocotyledonous flowering plants with hundreds of species, including the cultivated onion, garlic, scallion, shallot, leek, and chives. The generic name Allium is the Latin word for garlic, and the type species for the genus is Allium sativum which means "cultivated garlic".

Eric Block is an American chemist whose research has focused on the chemistry of organosulfur and organoselenium compounds, Allium chemistry, and the chemistry of olfaction. As of 2018, he is Distinguished Professor of Chemistry Emeritus at the University at Albany, SUNY.

Tetrahydrocannabiphorol (THCP) is a potent phytocannabinoid, a CB1 and CB2 agonist which was known as a synthetic homologue of THC, but for the first time in 2019 was isolated as a natural product in trace amounts from Cannabis sativa. It is structurally similar to Δ9-THC, the main active component of cannabis, but with the pentyl side chain extended to heptyl. Since it has a longer side chain, its cannabinoid effects are "far higher than Δ9-THC itself." Tetrahydrocannabiphorol has a reported binding affinity of 1.2 nM at CB1, approximately 33 times that of Δ9-THC (40 nM at CB1).

5-Methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile, also known as ROY (red-orange-yellow), is an organic compound which is a chemical intermediate to the drug olanzapine. It has been the subject of intensive study because it can exist in multiple well-characterised crystalline polymorphic forms.