
Liquid oxygen—abbreviated LOx, LOX, LOXygen or Lox in the aerospace, submarine and gas industries—is the liquid form of molecular oxygen. It was used as the oxidizer in the first liquid-fueled rocket invented in 1926 by Robert H. Goddard, an application which has continued to the present.

In quantum mechanics, an excited state of a system is any quantum state of the system that has a higher energy than the ground state. Excitation refers to an increase in energy level above a chosen starting point, usually the ground state, but sometimes an already excited state. The temperature of a group of particles is indicative of the level of excitation.

Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemical with the formula O=O (also written as 1
[O
2] or 1
O
2), which is in a quantum state where all electrons are spin paired. It is kinetically unstable at ambient temperature, but the rate of decay is slow.
Solid oxygen forms at normal atmospheric pressure at a temperature below 54.36 K (−218.79 °C, −361.82 °F). Solid oxygen O2, like liquid oxygen, is a clear substance with a light sky-blue color caused by absorption in the red part of the visible light spectrum.
TURBOMOLE is an ab initio computational chemistry program that implements various quantum chemistry methods. It was initially developed by the group of Prof. Reinhart Ahlrichs at the University of Karlsruhe. In 2007, TURBOMOLE GmbH, founded by R. Ahlrichs, F. Furche, C. Hättig, W. Klopper, M. Sierka, and F. Weigend, took over the responsibility for the coordination of the scientific development of TURBOMOLE program, for which the company holds all copy and intellectual property rights. In 2018 David P. Tew joined the TURBOMOLE GmbH. Since 1987, this program is one of the useful tools as it involves in many fields of research including heterogeneous and homogeneous catalysis, organic and inorganic chemistry, spectroscopy as well as biochemistry. This can be illustrated by citation records of Ahlrich's 1989 publication which is more than 6700 times as of 18 July 2020. In the year 2014, the second Turbomole article has been published. The number of citations from both papers indicates that the Turbomole's user base is expanding.
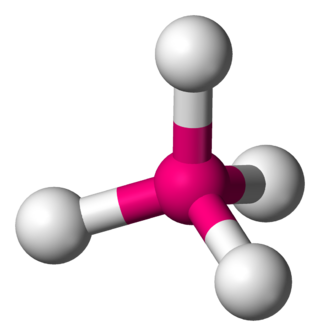
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1(−1⁄3) = 109.4712206...° ≈ 109.5° when all four substituents are the same, as in methane as well as its heavier analogues. Methane and other perfectly symmetrical tetrahedral molecules belong to point group Td, but most tetrahedral molecules have lower symmetry. Tetrahedral molecules can be chiral.

Carbon trioxide (CO3) is an unstable oxide of carbon (an oxocarbon). The possible isomers of carbon trioxide include ones with molecular symmetry point groups Cs, D3h, and C2v. The C2v state, consisting of a dioxirane, has been shown to be the ground state of the molecule. Carbon trioxide should not be confused with the stable carbonate ion (CO2−
3).

In computational chemistry, a water model is used to simulate and thermodynamically calculate water clusters, liquid water, and aqueous solutions with explicit solvent. The models are determined from quantum mechanics, molecular mechanics, experimental results, and these combinations. To imitate a specific nature of molecules, many types of models have been developed. In general, these can be classified by the following three points; (i) the number of interaction points called site, (ii) whether the model is rigid or flexible, (iii) whether the model includes polarization effects.

In chemistry, a water cluster is a discrete hydrogen bonded assembly or cluster of molecules of water. Many such clusters have been predicted by theoretical models (in silico), and some have been detected experimentally in various contexts such as ice, bulk liquid water, in the gas phase, in dilute mixtures with non-polar solvents, and as water of hydration in crystal lattices. The simplest example is the water dimer (H2O)2.
There are several known allotropes of oxygen. The most familiar is molecular oxygen, present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone. Others are:

Octaoxygen, also known as ε-oxygen or red oxygen, is an allotrope of oxygen consisting of eight oxygen atoms. This allotrope forms above 600 K at pressures greater than 17 GPa.

In chemistry, an oxocarbon or oxide of carbon is a chemical compound consisting only of carbon and oxygen. The simplest and most common oxocarbons are carbon monoxide (CO) and carbon dioxide. Many other stable or metastable oxides of carbon are known, but they are rarely encountered, such as carbon suboxide and mellitic anhydride.

Carbon pentaoxide, carbon pentoxide or tetraoxolan-5-one is an unstable molecular oxide of carbon. The molecule has been produced and studied at cryogenic temperatures. The molecule is important in atmospheric chemistry and in the study of cold ices in the outer solar system and interstellar space. The substance could form and be present on Ganymede or Triton, moons in the outer solar system.

Carbon hexoxide or carbon hexaoxide is an oxide of carbon with an unusually large quantity of oxygen. The molecule has been produced and studied at cryogenic temperatures. The molecule is important in atmospheric chemistry and in the study of cold ices in the outer solar system and interstellar space. The substance could form and be present on Ganymede or Triton, moons in the outer solar system. The molecule consists of a six membered ring with five oxygen and one carbon atom, and one oxygen with a double bond with the carbon.
Helium is the smallest and the lightest noble gas and one of the most unreactive elements, so it was commonly considered that helium compounds cannot exist at all, or at least under normal conditions. Helium's first ionization energy of 24.57 eV is the highest of any element. Helium has a complete shell of electrons, and in this form the atom does not readily accept any extra electrons nor join with anything to make covalent compounds. The electron affinity is 0.080 eV, which is very close to zero. The helium atom is small with the radius of the outer electron shell at 0.29 Å. Helium is a very hard atom with a Pearson hardness of 12.3 eV. It has the lowest polarizability of any kind of atom, however, very weak van der Waals forces exist between helium and other atoms. This force may exceed repulsive forces, so at extremely low temperatures helium may form van der Waals molecules. Helium has the lowest boiling point of any known substance.
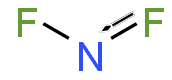
Nitrogen difluoride, also known as difluoroamino, is a reactive radical molecule with formula NF2. This small molecule is in equilibrium with its dimer dinitrogen tetrafluoride.

Solid nitrogen is a number of solid forms of the element nitrogen, first observed in 1884. Solid nitrogen is mainly the subject of academic research, but low-temperature, low-pressure solid nitrogen is a substantial component of bodies in the outer Solar System and high-temperature, high-pressure solid nitrogen is a powerful explosive, with higher energy density than any other non-nuclear material.
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space. One solid interstitial compound of argon, Ar1C60 is stable at room temperature. Ar1C60 was discovered by the CSIRO.
The magnesium argide ion, MgAr+ is an ion composed of one ionised magnesium atom, Mg+ and an argon atom. It is important in inductively coupled plasma mass spectrometry and in the study of the field around the magnesium ion. The ionization potential of magnesium is lower than the first excitation state of argon, so the positive charge in MgAr+ will reside on the magnesium atom. Neutral MgAr molecules can also exist in an excited state.
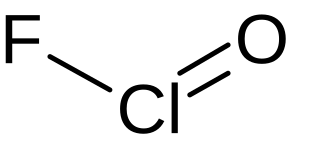
Chlorosyl fluoride is an inorganic compound of chlorine, fluorine, and oxygen with the chemical formula OClF.
















