Related Research Articles

Neutron activation analysis (NAA) is a nuclear process used for determining the concentrations of elements in many materials. NAA allows discrete sampling of elements as it disregards the chemical form of a sample, and focuses solely on atomic nuclei. The method is based on neutron activation and thus requires a neutron source. The sample is bombarded with neutrons, causing its constituent elements to form radioactive isotopes. The radioactive emissions and radioactive decay paths for each element have long been studied and determined. Using this information, it is possible to study spectra of the emissions of the radioactive sample, and determine the concentrations of the various elements within it. A particular advantage of this technique is that it does not destroy the sample, and thus has been used for the analysis of works of art and historical artifacts. NAA can also be used to determine the activity of a radioactive sample.
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (t1/2) for that collection, can be calculated from their measured decay constants. The range of the half-lives of radioactive atoms has no known limits and spans a time range of over 55 orders of magnitude.
A synthetic radioisotope is a radionuclide that is not found in nature: no natural process or mechanism exists which produces it, or it is so unstable that it decays away in a very short period of time. Examples include technetium-99 and promethium-146. Many of these are found in, and harvested from, spent nuclear fuel assemblies. Some must be manufactured in particle accelerators.
A radioactive tracer, radiotracer, or radioactive label is a synthetic derivative of a natural compound in which one or more atoms have been replaced by a radionuclide. By virtue of its radioactive decay, it can be used to explore the mechanism of chemical reactions by tracing the path that the radioisotope follows from reactants to products. Radiolabeling or radiotracing is thus the radioactive form of isotopic labeling. In biological contexts, experiments that use radioisotope tracers are sometimes called radioisotope feeding experiments.
An atomic battery, nuclear battery, radioisotope battery or radioisotope generator is a device which uses energy from the decay of a radioactive isotope to generate electricity. Like nuclear reactors, they generate electricity from nuclear energy, but differ in that they do not use a chain reaction. Although commonly called batteries, they are technically not electrochemical and cannot be charged or recharged. They are very costly, but have an extremely long life and high energy density, and so they are typically used as power sources for equipment that must operate unattended for long periods of time, such as spacecraft, pacemakers, underwater systems and automated scientific stations in remote parts of the world.
Fluorine (9F) has 18 known isotopes ranging from 13
F
to 31
F
and two isomers. Only fluorine-19 is stable and naturally occurring in more than trace quantities; therefore, fluorine is a monoisotopic and mononuclidic element.

Thallium (81Tl) has 41 isotopes with atomic masses that range from 176 to 216. 203Tl and 205Tl are the only stable isotopes and 204Tl is the most stable radioisotope with a half-life of 3.78 years. 207Tl, with a half-life of 4.77 minutes, has the longest half-life of naturally occurring Tl radioisotopes. All isotopes of thallium are either radioactive or observationally stable, meaning that they are predicted to be radioactive but no actual decay has been observed.
Naturally occurring thulium (69Tm) is composed of one stable isotope, 169Tm. Thirty-nine radioisotopes have been characterized, with the most stable being 171Tm with a half-life of 1.92 years, 170Tm with a half-life of 128.6 days, 168Tm with a half-life of 93.1 days, and 167Tm with a half-life of 9.25 days. All of the remaining radioactive isotopes have half-lives that are less than 64 hours, and the majority of these have half-lives that are less than 2 minutes. This element also has 26 meta states, with the most stable being 164mTm, 160mTm and 155mTm.

There are 37 known isotopes of iodine (53I) from 108I to 144I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element.
Technetium (43Tc) is one of the two elements with Z < 83 that have no stable isotopes; the other such element is promethium. It is primarily artificial, with only trace quantities existing in nature produced by spontaneous fission or neutron capture by molybdenum. The first isotopes to be synthesized were 97Tc and 99Tc in 1936, the first artificial element to be produced. The most stable radioisotopes are 97Tc, 98Tc, and 99Tc.
Naturally occurring zirconium (40Zr) is composed of four stable isotopes (of which one may in the future be found radioactive), and one very long-lived radioisotope (96Zr), a primordial nuclide that decays via double beta decay with an observed half-life of 2.0×1019 years; it can also undergo single beta decay, which is not yet observed, but the theoretically predicted value of t1/2 is 2.4×1020 years. The second most stable radioisotope is 93Zr, which has a half-life of 1.53 million years. Thirty other radioisotopes have been observed. All have half-lives less than a day except for 95Zr (64.02 days), 88Zr (83.4 days), and 89Zr (78.41 hours). The primary decay mode is electron capture for isotopes lighter than 92Zr, and the primary mode for heavier isotopes is beta decay.
Neptunium (93Np) is usually considered an artificial element, although trace quantities are found in nature, so a standard atomic weight cannot be given. Like all trace or artificial elements, it has no stable isotopes. The first isotope to be synthesized and identified was 239Np in 1940, produced by bombarding 238
U
with neutrons to produce 239
U
, which then underwent beta decay to 239
Np
.
Plutonium (94Pu) is an artificial element, except for trace quantities resulting from neutron capture by uranium, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no stable isotopes. It was synthesized long before being found in nature, the first isotope synthesized being plutonium-238 in 1940. Twenty plutonium radioisotopes have been characterized. The most stable are plutonium-244 with a half-life of 80.8 million years; plutonium-242 with a half-life of 373,300 years; and plutonium-239 with a half-life of 24,110 years; and plutonium-240 with a half-life of 6,560 years. This element also has eight meta states; all have half-lives of less than one second.
Radionuclides which emit gamma radiation are valuable in a range of different industrial, scientific and medical technologies. This article lists some common gamma-emitting radionuclides of technological importance, and their properties.
Various radionuclides emit beta particles, high-speed electrons or positrons, through radioactive decay of their atomic nucleus. These can be used in a range of different industrial, scientific, and medical applications. This article lists some common beta-emitting radionuclides of technological importance, and their properties.
Iodine-125 (125I) is a radioisotope of iodine which has uses in biological assays, nuclear medicine imaging and in radiation therapy as brachytherapy to treat a number of conditions, including prostate cancer, uveal melanomas, and brain tumors. It is the second longest-lived radioisotope of iodine, after iodine-129.
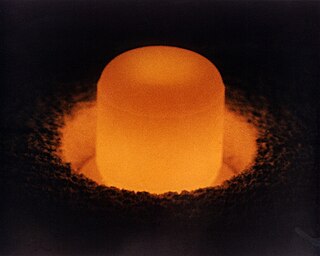
Plutonium-238 is a radioactive isotope of plutonium that has a half-life of 87.7 years.
Iodine-123 (123I) is a radioactive isotope of iodine used in nuclear medicine imaging, including single-photon emission computed tomography (SPECT) or SPECT/CT exams. The isotope's half-life is 13.2230 hours; the decay by electron capture to tellurium-123 emits gamma radiation with a predominant energy of 159 keV. In medical applications, the radiation is detected by a gamma camera. The isotope is typically applied as iodide-123, the anionic form.
Gold-198 (198Au) is a radioactive isotope of gold. It undergoes beta decay to stable 198Hg with a half-life of 2.69464 days.

Peptide receptor radionuclide therapy (PRRT) is a type of radionuclide therapy, using a radiopharmaceutical that targets peptide receptors to deliver localised treatment, typically for neuroendocrine tumours (NETs).
References
- 1 2 3 4 5 Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- 1 2 3 "NuDat 3". www.nndc.bnl.gov.
- 1 2 3 Polyak, Andras; Das, Tapas; Chakraborty, Sudipta; Kiraly, Reka; Dabasi, Gabriella; Joba, Robert Peter; Jakab, Csaba; Thuroczy, Julianna; Postenyi, Zita; Haasz, Veronika; Janoki, Gergely; Janoki, Gyozo A.; Pillai, Maroor R.A.; Balogh, Lajos (October 2014). "Thulium-170-Labeled Microparticles for Local Radiotherapy: Preliminary Studies". Cancer Biotherapy and Radiopharmaceuticals. 29 (8): 330–338. doi:10.1089/cbr.2014.1680. ISSN 1084-9785. PMID 25226213 – via Academia.edu.
- ↑ Mertzimekis, Theo J. "NUMOR | Nuclear Moments and Radii | University of Athens | since 2007". magneticmoments.info. Retrieved 12 November 2023.
- ↑ Walter, C.E.; Van Konynenburg, R.; VanSant, J.H. (6 September 1990). "Thulium-170 heat source". doi:10.2172/10156110. OSTI 10156110.
- 1 2 3 Dustin, J. Seth; Borrelli, R.A. (December 2021). "Assessment of alternative radionuclides for use in a radioisotope thermoelectric generator". Nuclear Engineering and Design. 385: 111475. doi: 10.1016/j.nucengdes.2021.111475 . S2CID 240476644.
- ↑ Hilbish, Theodore F. (November 1954). "Developments in diagnostic radiology". Public Health Reports. 69 (11): 1017–1027. doi:10.2307/4588947. ISSN 0094-6214. JSTOR 4588947. PMC 2024396 . PMID 13215708.
- ↑ Halmshaw, Ronald (1995). Industrial radiology: theory and practice (2. ed.). London: Chapman & Hall. pp. 59–60. ISBN 0412627809.
- ↑ Vats, Kusum; Das, Tapas; Sarma, Haladhar D.; Banerjee, Sharmila; Pillai, M.r.a. (December 2013). "Radiolabeling, Stability Studies, and Pharmacokinetic Evaluation of Thulium-170-Labeled Acyclic and Cyclic Polyaminopolyphosphonic Acids" (PDF). Cancer Biotherapy and Radiopharmaceuticals. 28 (10): 737–745. doi:10.1089/cbr.2013.1475. ISSN 1084-9785. PMID 23931111. Archived from the original (PDF) on 2023-11-12.
- ↑ Walter, C. E. (1 July 1991). "Infrastructure for thulium-170 isotope power systems for autonomous underwater vehicle fleets". Lawrence Livermore National Lab., CA (United States). OSTI 5491258.
- ↑ Alderman, Carol J. (1993). "Thulium heat sources for space power application". AIP Conference Proceedings. Vol. 271. pp. 1085–1091. doi:10.1063/1.43194.