Related Research Articles

Emergency contraception (EC) is a birth control measure, used after sexual intercourse to prevent pregnancy.

The diaphragm is a barrier method of birth control. It is moderately effective, with a one-year failure rate of around 12% with typical use. It is placed over the cervix with spermicide before sex and left in place for at least six hours after sex. Fitting by a healthcare provider is generally required.

Nonoxynol-9, sometimes abbreviated as N-9, is an organic compound that is used as a surfactant. It is a member of the nonoxynol family of nonionic surfactants. N-9 and related compounds are ingredients in various cleaning and cosmetic products. It is widely used in contraceptives for its spermicidal properties.
Spermicide is a contraceptive substance that destroys sperm, inserted vaginally prior to intercourse to prevent pregnancy. As a contraceptive, spermicide may be used alone. However, the pregnancy rate experienced by couples using only spermicide is higher than that of couples using other methods. Usually, spermicides are combined with contraceptive barrier methods such as diaphragms, condoms, cervical caps, and sponges. Combined methods are believed to result in lower pregnancy rates than either method alone.

The cervical cap is a form of barrier contraception. A cervical cap fits over the cervix and blocks sperm from entering the uterus through the external orifice of the uterus, called the os.

A menstrual cup is a menstrual hygiene device which is inserted into the vagina during menstruation. Its purpose is to collect menstrual fluid. Menstrual cups are made of elastomers. A properly-fitting menstrual cup seals against the vaginal walls, so tilting and inverting the body will not cause it to leak. It is impermeable and collects menstrual fluid, unlike tampons and menstrual pads, which absorb it.

The contraceptive sponge combines barrier and spermicidal methods to prevent conception. Sponges work in two ways. First, the sponge is inserted into the vagina, so it can cover the cervix and prevent any sperm from entering the uterus. Secondly, the sponge contains spermicide.

Levonorgestrel is a hormonal medication which is used in a number of birth control methods. It is combined with an estrogen to make combination birth control pills. As an emergency birth control, sold under the brand names Plan B One-Step and Julie, among others, it is useful within 72 hours of unprotected sex. The more time that has passed since sex, the less effective the medication becomes, and it does not work after pregnancy (implantation) has occurred. Levonorgestrel works by preventing ovulation or fertilization from occurring. It decreases the chances of pregnancy by 57–93%. In an intrauterine device (IUD), such as Mirena among others, it is effective for the long-term prevention of pregnancy. A levonorgestrel-releasing implant is also available in some countries.
Levonorgestrel-releasing implant, sold under the brand name Jadelle among others, are devices that release levonorgestrel for birth control. It is one of the most effective forms of birth control with a one-year failure rate around 0.05%. The device is placed under the skin and lasts for up to five years. It may be used by women who have a history of pelvic inflammatory disease and therefore cannot use an intrauterine device. Following removal, fertility quickly returns.

A contraceptive patch, also known as "the patch", is a transdermal patch applied to the skin that releases synthetic oestrogen and progestogen hormones to prevent pregnancy. They have been shown to be as effective as the combined oral contraceptive pill with perfect use, and the patch may be more effective in typical use.
Extended or continuous cycle combined oral contraceptive pills are a packaging of combined oral contraceptive pills (COCPs) that reduce or eliminate the withdrawal bleeding that would occur once every 28 days in traditionally packaged COCPs. It works by reducing the frequency of the pill-free or placebo days. Extended cycle use of COCPs may also be called menstrual suppression, although other hormonal medications or medication delivery systems may also be used to suppress menses. Any brand of combined oral contraceptive pills can be used in an extended or continuous manner by simply discarding the placebo pills; this is most commonly done with monophasic pills in which all of the pills in a package contain the same fixed dosing of a synthetic estrogen and a progestin in each active pill.
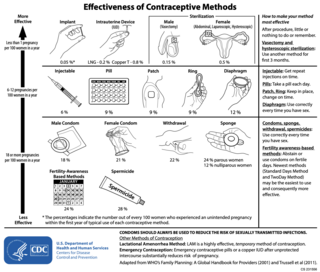
There are many methods of birth control that vary in requirements, side effects, and effectiveness. As the technology, education, and awareness about contraception has evolved, new contraception methods have been theorized and put in application. Although no method of birth control is ideal for every user, some methods remain more effective, affordable or intrusive than others. Outlined here are the different types of barrier methods, hormonal methods, various methods including spermicides, emergency contraceptives, and surgical methods and a comparison between them.
Ortho Pharmaceutical was initially formed in the United States in 1931 as a subsidiary of Johnson & Johnson to market the first prescription spermicidal contraceptive jelly, Ortho-Gynol.

Mestranol/norethynodrel was the first combined oral contraceptive pill (COCP) being mestranol and norethynodrel. It sold as Enovid in the United States and as Enavid in the United Kingdom. Developed by Gregory Pincus at G. D. Searle & Company, it was first approved on June 10, 1957, by the U.S. Food and Drug Administration for treatment of menstrual disorders. The FDA approved an additional indication for use as a contraceptive on June 23, 1960, though it only became legally prescribable nationwide and regardless of the woman's marital status after Eisenstadt v. Baird in 1972. In 1961, it was approved as a contraceptive in the UK and in Canada.
Mentor Worldwide LLC is an American company that supplies surgical aesthetics products to plastic surgeons. The company is based in Santa Barbara, California. It produces one of two silicone gel breast implants. Titled MemoryGel, the product was approved by the U.S. Food and Drug Administration (FDA) on November 17, 2006. The other FDA-approved products are developed by competitors Allergan and Sientra. Mentor also produces a range of lipoplasty equipment for liposuction procedures as well as a Niacin-based skincare product line called NIA 24.

Birth control, also known as contraception, anticonception, and fertility control, is the use of methods or devices to prevent unintended pregnancy. Birth control has been used since ancient times, but effective and safe methods of birth control only became available in the 20th century. Planning, making available, and using human birth control is called family planning. Some cultures limit or discourage access to birth control because they consider it to be morally, religiously, or politically undesirable.

The womb veil was a 19th-century American form of barrier contraception consisting of an occlusive pessary, i.e. a device inserted into the vagina to block access of the sperm into the uterus. Made of rubber, it was a forerunner to the modern diaphragm and cervical cap. The name was first used by Edward Bliss Foote in 1863 for the device he designed and marketed. "Womb veil" became the most common 19th-century American term for similar devices, and continued to be used into the early 20th century. Womb veils were among a "range of contraceptive technology of questionable efficacy" available to American women of the 19th century, forms of which began to be advertised in the 1830s and 1840s. They could be bought widely through mail-order catalogues; when induced abortion was criminalized during the 1870s, reliance on birth control increased. Womb veils were touted as a discreet form of contraception, with one catalogue of erotic products from the 1860s promising that they could be "used by the female without danger of detection by the male."

Wyeth was a pharmaceutical company until it was purchased by Pfizer in 2009. The company was founded in Philadelphia, Pennsylvania, in 1860 as John Wyeth and Brother. Its headquarters moved to Collegeville, Pennsylvania, and Madison, New Jersey, before its headquarters were consolidated with Pfizer's in New York City after the 2009 merger.

CONRAD is a non-profit organization scientific research organization that works to improve the reproductive health of women, especially in developing countries. CONRAD was established in 1986 under a cooperative agreement between Eastern Virginia Medical School (EVMS) and the United States Agency for International Development(USAID). CONRAD’s products are developed primarily for women in low-resource settings, in that they are designed to be safe, affordable and user-friendly. CONRAD is led by Scientific and Executive Director Gustavo Doncel, M.D., Ph.D. Primary funding for CONRAD comes from the U.S. President's Emergency Plan for AIDS Relief (PEPFAR) through the U.S. Agency for International Development (USAID), with additional funding from The Bill & Melinda Gates Foundation and the National Institutes of Health (NIH).
Combined hormonal contraception (CHC), or combined birth control, is a form of hormonal contraception which combines both an estrogen and a progestogen in varying formulations.
References
- ↑ Lockerm, Sari (2005). Today Sponge. The Complete Idiot's Guide to Amazing Sex, p. 367. Penguin, ISBN 978-1-59257-327-1
- ↑ Day, Kathleen (January 22, 1985). VLI to Begin Marketing `Today' Sponge in Britain. Los Angeles Times
- 1 2 "Out of Stock". todaysponge.com. 2019. Archived from the original on April 26, 2010. Retrieved 20 May 2023.
- ↑ Emmons, Steve (February 15, 1993). The final fall. Los Angeles Times
- ↑ FDA's approval of the Today contraceptive sponge: hearing before a subcommittee of the Committee on Government Operations, House of Representatives, Ninety-eighth Congress, first session, July 13, 1983
- ↑ Granelli, James S. (October 19, 1985). VLI Reports. Los Angeles Times
- ↑ Day, Kathleeen (November 13, 1984). VLI Contraceptive Sponge 'Relatively Safe,' Says FDA. Los Angeles Times
- ↑ Millenson, Michael L. (January 12, 1984). Sales soar for firm's Today sponge contraceptives. Chicago Tribune
- ↑ Edelman DA, McIntyre SL, Harper J (1984). A comparative trial of the Today contraceptive sponge and diaphragm. American Journal of Obstetrics and Gynecology 1984 Dec 1;150(7):869-76. PMID 6095664
- ↑ Edelman DA, North BB (1987). Parity and the effectiveness of the Today contraceptive sponge. Adv Contracept. 1987 Dec;3(4):327-33. PMID 3445801
- ↑ Berger KL, Remington K (1987). The prophylactic properties of the Today sponge and other spermicide containing contraceptives. Adv Contracept. 1987 Jun;3(2):125-31. PMID 2820207
- ↑ Schuiling, Kerri Durnell and Likis, Frances E. (2006). Jones & Bartlett Learning, ISBN 978-0-7637-4717-6
- ↑ Associated Press (December 7, 1983). Birth control device linked to toxic shock. The Free Lance-Star
- ↑ Rosenthal MS (2003). The Gynecological Sourcebook, p. 144. McGraw-Hill, ISBN 978-0-07-140279-8
- ↑ George, John (2007-01-16). "Synova Healthcare finds company worthy of purchase". Philadelphia Business Journal. Retrieved 2007-01-16.
- ↑ "Synova Healthcare, maker of contraceptive sponge filed for bankruptcy". December 19, 2007.
- ↑ "Today Sponge Returns To Store Shelves". Mayer Laboratories, Inc. 2009. Retrieved 2009-05-14.
- ↑ "Todays® Sponge Returns To Store Shelves". Alvogen, Inc. 2009. Retrieved 2022-09-22.
- ↑ "Mayer Labs Acquires Today(R) Sponge Distribution Rights". Biospace.com. 2009. Retrieved 2022-09-25.
- ↑ Lavery, David and Sara Lewis Dunne (2006). Seinfeld, master of its domain: revisiting television's greatest sitcom, p. 247. Continuum International Publishing Group, ISBN 978-0-8264-1803-6