
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two organyl groups. They have the general formula R−O−R′, where R and R′ represent organyl groups. Ethers can again be classified into two varieties: if the organyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether". Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

Acetoacetic acid is the organic compound with the formula CH3COCH2COOH. It is the simplest beta-keto acid, and like other members of this class, it is unstable. The methyl and ethyl esters, which are quite stable, are produced on a large scale industrially as precursors to dyes. Acetoacetic acid is a weak acid.
In organic chemistry, the Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide (DMSO) and an organic base, such as triethylamine. It is one of the many oxidation reactions commonly referred to as 'activated DMSO' oxidations. The reaction is known for its mild character and wide tolerance of functional groups.

In organic chemistry, an oxime is an organic compound belonging to the imines, with the general formula RR’C=N−OH, where R is an organic side-chain and R' may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides with general structure R1C(=NOH)NR2R3.

Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene. Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. They are used as protecting groups of carbonyl groups, making them essential in synthesis of organic chemistry.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.

Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula NaBH4. It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodium borohydride is a reducing agent that finds application in papermaking and dye industries. It is also used as a reagent in organic synthesis.
Organoselenium chemistry is the science exploring the properties and reactivity of organoselenium compounds, chemical compounds containing carbon-to-selenium chemical bonds. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments.
The Strecker amino acid synthesis, also known simply as the Strecker synthesis, is a method for the synthesis of amino acids by the reaction of an aldehyde with cyanide in the presence of ammonia. The condensation reaction yields an α-aminonitrile, which is subsequently hydrolyzed to give the desired amino acid. The method is used for the commercial production of racemic methionine from methional.
Organophosphorus chemistry is the scientific study of the synthesis and properties of organophosphorus compounds, which are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents.

Grignard reagents or Grignard compounds are chemical compounds with the general formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH3 and phenylmagnesium bromide (C6H5)−Mg−Br. They are a subclass of the organomagnesium compounds.
In chemistry, transfer hydrogenation is a chemical reaction involving the addition of hydrogen to a compound from a source other than molecular H2. It is applied in laboratory and industrial organic synthesis to saturate organic compounds and reduce ketones to alcohols, and imines to amines. It avoids the need for high-pressure molecular H2 used in conventional hydrogenation. Transfer hydrogenation usually occurs at mild temperature and pressure conditions using organic or organometallic catalysts, many of which are chiral, allowing efficient asymmetric synthesis. It uses hydrogen donor compounds such as formic acid, isopropanol or dihydroanthracene, dehydrogenating them to CO2, acetone, or anthracene respectively. Often, the donor molecules also function as solvents for the reaction. A large scale application of transfer hydrogenation is coal liquefaction using "donor solvents" such as tetralin.

The Stork enamine alkylation involves the addition of an enamine to a Michael acceptor or another electrophilic alkylation reagent to give an alkylated iminium product, which is hydrolyzed by dilute aqueous acid to give the alkylated ketone or aldehyde. Since enamines are generally produced from ketones or aldehydes, this overall process constitutes a selective monoalkylation of a ketone or aldehyde, a process that may be difficult to achieve directly.

The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of alcohols. Its use has subsided because milder, more selective reagents have been developed, e.g. Collins reagent.
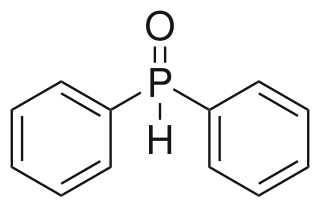
Diphenylphosphine oxide is an organophosphorus compound with the formula (C6H5)2P(O)H. It is a white solid that soluble in polar organic solvents.

Diethyl phosphite is the organophosphorus compound with the formula (C2H5O)2P(O)H. It is a popular reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. Diethyl phosphite is a colorless liquid. The molecule is tetrahedral.















