
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation. A perceptible example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the electromagnetic spectrum, while the emitted light is in the visible region; this gives the fluorescent substance a distinct color that can only be seen when the substance has been exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after.

Photoluminescence is light emission from any form of matter after the absorption of photons. It is one of many forms of luminescence and is initiated by photoexcitation, hence the prefix photo-. Following excitation, various relaxation processes typically occur in which other photons are re-radiated. Time periods between absorption and emission may vary: ranging from short femtosecond-regime for emission involving free-carrier plasma in inorganic semiconductors up to milliseconds for phosphoresence processes in molecular systems; and under special circumstances delay of emission may even span to minutes or hours.

Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluorescence, a phosphorescent material does not immediately reemit the radiation it absorbs. Instead, a phosphorescent material absorbs some of the radiation energy and reemits it for a much longer time after the radiation source is removed.

Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet, visible light (400–750 nm) or infrared radiation (750–2500 nm).

A scintillator is a material that exhibits scintillation, the property of luminescence, when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate. Sometimes, the excited state is metastable, so the relaxation back down from the excited state to lower states is delayed. The process then corresponds to one of two phenomena: delayed fluorescence or phosphorescence. The correspondence depends on the type of transition and hence the wavelength of the emitted optical photon.

Fluorescence spectroscopy is a type of electromagnetic spectroscopy that analyzes fluorescence from a sample. It involves using a beam of light, usually ultraviolet light, that excites the electrons in molecules of certain compounds and causes them to emit light; typically, but not necessarily, visible light. A complementary technique is absorption spectroscopy. In the special case of single molecule fluorescence spectroscopy, intensity fluctuations from the emitted light are measured from either single fluorophores, or pairs of fluorophores.

In quantum mechanics, an excited state of a system is any quantum state of the system that has a higher energy than the ground state. Excitation refers to an increase in energy level above a chosen starting point, usually the ground state, but sometimes an already excited state. The temperature of a group of particles is indicative of the level of excitation.
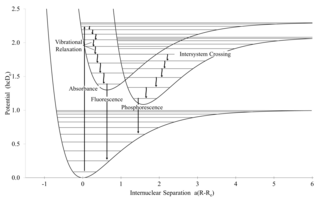
Intersystem crossing (ISC) is an isoenergetic radiationless process involving a transition between the two electronic states with different spin multiplicity.
In physics and physical chemistry, time-resolved spectroscopy is the study of dynamic processes in materials or chemical compounds by means of spectroscopic techniques. Most often, processes are studied after the illumination of a material occurs, but in principle, the technique can be applied to any process that leads to a change in properties of a material. With the help of pulsed lasers, it is possible to study processes that occur on time scales as short as 10−16 seconds. All time-resolved spectra are suitable to be analyzed using the two-dimensional correlation method for a correlation map between the peaks.

A fluorophore is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds.

Stokes shift is the difference between positions of the band maxima of the absorption and emission spectra of the same electronic transition. It is named after Irish physicist George Gabriel Stokes. Sometimes Stokes shifts are given in wavelength units, but this is less meaningful than energy, wavenumber or frequency units because it depends on the absorption wavelength. For instance, a 50 nm Stokes shift from absorption at 300 nm is larger in terms of energy than a 50 nm Stokes shift from absorption at 600 nm.
In particle physics, the quantum yield of a radiation-induced process is the number of times a specific event occurs per photon absorbed by the system.
Phosphorescent organic light-emitting diodes (PHOLED) are a type of organic light-emitting diode (OLED) that use the principle of phosphorescence to obtain higher internal efficiencies than fluorescent OLEDs. This technology is currently under development by many industrial and academic research groups.

Photosensitizers are light absorbers that alters the course of a photochemical reaction. They usually are catalysts. They can function by many mechanisms, sometimes they donate an electron to the substrate, sometimes they abstract a hydrogen atom from the substrate. At the end of this process, the photosensitizer returns to its ground state, where it remains chemically intact, poised to absorb more light. One branch of chemistry which frequently utilizes photosensitizers is polymer chemistry, using photosensitizers in reactions such as photopolymerization, photocrosslinking, and photodegradation. Photosensitizers are also used to generate prolonged excited electronic states in organic molecules with uses in photocatalysis, photon upconversion and photodynamic therapy. Generally, photosensitizers absorb electromagnetic radiation consisting of infrared radiation, visible light radiation, and ultraviolet radiation and transfer absorbed energy into neighboring molecules. This absorption of light is made possible by photosensitizers' large de-localized π-systems, which lowers the energy of HOMO and LUMO orbitals to promote photoexcitation. While many photosensitizers are organic or organometallic compounds, there are also examples of using semiconductor quantum dots as photosensitizers.

Photoexcitation is the production of an excited state of a quantum system by photon absorption. The excited state originates from the interaction between a photon and the quantum system. Photons carry energy that is determined by the wavelengths of the light that carries the photons. Objects that emit light with longer wavelengths, emit photons carrying less energy. In contrast to that, light with shorter wavelengths emit photons with more energy. When the photon interacts with a quantum system, it is therefore important to know what wavelength one is dealing with. A shorter wavelength will transfer more energy to the quantum system than longer wavelengths.

Fluorescence is used in the life sciences generally as a non-destructive way of tracking or analysing biological molecules. Some proteins or small molecules in cells are naturally fluorescent, which is called intrinsic fluorescence or autofluorescence. Alternatively, specific or general proteins, nucleic acids, lipids or small molecules can be "labelled" with an extrinsic fluorophore, a fluorescent dye which can be a small molecule, protein or quantum dot. Several techniques exist to exploit additional properties of fluorophores, such as fluorescence resonance energy transfer, where the energy is passed non-radiatively to a particular neighbouring dye, allowing proximity or protein activation to be detected; another is the change in properties, such as intensity, of certain dyes depending on their environment allowing their use in structural studies.
Photoelectrochemical processes are processes in photoelectrochemistry; they usually involve transforming light into other forms of energy. These processes apply to photochemistry, optically pumped lasers, sensitized solar cells, luminescence, and photochromism.

Photon upconversion (UC) is a process in which the sequential absorption of two or more photons leads to the emission of light at shorter wavelength than the excitation wavelength. It is an anti-Stokes type emission. An example is the conversion of infrared light to visible light. Upconversion can take place in both organic and inorganic materials, through a number of different mechanisms. Organic molecules that can achieve photon upconversion through triplet-triplet annihilation are typically polycyclic aromatic hydrocarbons (PAHs). Inorganic materials capable of photon upconversion often contain ions of d-block or f-block elements. Examples of these ions are Ln3+, Ti2+, Ni2+, Mo3+, Re4+, Os4+, and so on.
Upconverting nanoparticles (UCNPs) are nanoscale particles that exhibit photon upconversion. In photon upconversion, two or more incident photons of relatively low energy are absorbed and converted into one emitted photon with higher energy. Generally, absorption occurs in the infrared, while emission occurs in the visible or ultraviolet regions of the electromagnetic spectrum. UCNPs are usually composed of rare-earth based lanthanide- or actinide-doped transition metals and are of particular interest for their applications in in vivo bio-imaging, bio-sensing, and nanomedicine because of their highly efficient cellular uptake and high optical penetrating power with little background noise in the deep tissue level. They also have potential applications in photovoltaics and security, such as infrared detection of hazardous materials.
Thermally activated delayed fluorescence (TADF) is a process through which a molecular species in a non-emitting excited state can incorporate surrounding thermal energy to change states and only then undergo light emission. The TADF process involves an excited molecular species in a triplet state, which commonly has a forbidden transition to the ground state termed phosphorescence. By absorbing nearby thermal energy the triplet state can undergo reverse intersystem crossing (RISC) converting it to a singlet state, which can then de-excite to the ground state and emit light in a process termed fluorescence. Along with fluorescent and phosphorescent compounds, TADF compounds are one of the three main light-emitting materials used in organic light-emitting diodes (OLEDs).















