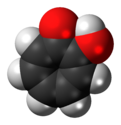
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (−OH) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, C
6H
5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.

L-Tyrosine or tyrosine or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek tyrós, meaning cheese, as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is encoded by the codons UAC and UAU in messenger RNA.
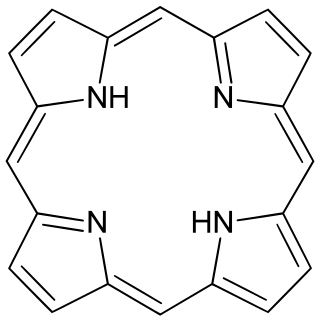
Porphyrins are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). In vertebrates, an essential member of the porphyrin group is heme, which is a component of hemoproteins, whose functions include carrying oxygen in the bloodstream. In plants, an essential porphyrin derivative is chlorophyll, which is involved in light harvesting and electron transfer in photosynthesis.
Phloroglucinol is an organic compound with the formula C6H3(OH)3. It is a colorless solid. It is used in the synthesis of pharmaceuticals and explosives. Phloroglucinol is one of three isomeric benzenetriols. The other two isomers are hydroxyquinol (1,2,4-benzenetriol) and pyrogallol (1,2,3-benzenetriol). Phloroglucinol, and its benzenetriol isomers, are still defined as "phenols" according to the IUPAC official nomenclature rules of chemical compounds. Many such monophenolics are often termed polyphenols.
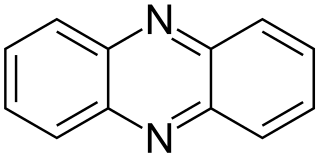
Phenazine is an organic compound with the formula (C6H4)2N2. It is a dibenzo annulated pyrazine, and the parent substance of many dyestuffs, such as the toluylene red, indulines, and safranines (and the closely related eurhodines). Phenazine crystallizes in yellow needles, which are only sparingly soluble in alcohol. Sulfuric acid dissolves it, forming a deep-red solution.

The Dakin oxidation (or Dakin reaction) is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde (2-hydroxybenzaldehyde or 4-hydroxybenzaldehyde) or ketone reacts with hydrogen peroxide (H2O2) in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidised, whereas the H2O2 is reduced.

Ralph Alexander Raphael was a British organic chemist, well known for his use of acteylene derivatives in the synthesis of natural products with biological activity.

A boronic acid is an organic compound related to boric acid in which one of the three hydroxyl groups is replaced by an alkyl or aryl group. As a compound containing a carbon–boron bond, members of this class thus belong to the larger class of organoboranes.

Tropone or 2,4,6-cycloheptatrien-1-one is an organic compound with some importance in organic chemistry as a non-benzenoid aromatic. The compound consists of a ring of seven carbon atoms with three conjugated alkene groups and a ketone group. The related compound tropolone (2-hydroxy-2,4,6-cycloheptatrien-1-one) has an additional alcohol group next to the ketone. Tropones are uncommon in natural products, with the notable exception of the 2-hydroxyl derivatives, which are called tropolones.
In organic chemistry, the Baudisch reaction is a process for the synthesis of nitrosophenols using metal ions. Although the products are of limited value, the reaction is of historical interest as an example of metal-promoted functionalization of aromatic substrates.

2-Ethylhexanoic acid is the organic compound with the formula CH3(CH2)3CH(C2H5)CO2H. It is a carboxylic acid that is widely used to prepare lipophilic metal derivatives that are soluble in nonpolar organic solvents. 2-Ethylhexanoic acid is a colorless viscous oil. It is supplied as a racemic mixture.

Pancratistatin (PST) is a natural compound initially extracted from spider lily, a Hawaiian native plant of the family Amaryllidaceae (AMD).

Indole is an organic compound with the formula C6H4CCNH3. Indoles are derivatives of indole where one or more H's have been replaced by other groups. Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are widely distributed in nature, most notably as amino acid tryptophan and neurotransmitter serotonin.

Thujaplicin is any of three isomeric tropolone-related natural products that have been isolated from the softwoods of the trees of Cupressaceae family. These compounds are known for their antibacterial, antifungal, and antioxidant properties. They were the first natural tropolones to be made synthetically.

Hinokitiol (β-thujaplicin) is a natural monoterpenoid found in the wood of trees in the family Cupressaceae. It is a tropolone derivative and one of the thujaplicins. Hinokitiol is used in oral and skin care products, and is a food additive used in Japan.
The Markó–Lam deoxygenation is an organic chemistry reaction where the hydroxy functional group in an organic compound is replaced by a hydrogen atom to give an alkyl group. The Markó-Lam reaction is a variant of the Bouveault–Blanc reduction and an alternative to the classical Barton–McCombie deoxygenation. It is named for the Belgian chemists István Markó and Kevin Lam.

Spinochrome E is a polyhydroxylated 1,4-naphthoquinone pigment found in sea urchin shell ("test"), spine, gonads, coelomic fluid, and eggs, of sea urchin commonly known as spinochromes. These natural phenolic compounds are quinones with potential pharmacological properties. The several hydroxyl groups are appropriate for free-radical scavenging, which diminishes ROS and prevents redox imbalance. Mechanisms are described such as scavenging of reactive oxygen species (ROS), interaction with lipid peroxide radicals, chelation of metal ions, inhibition of lipid peroxidation and regulation of the cell redox potential.
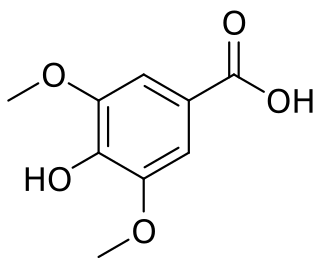
Syringic acid is a naturally occurring phenolic compound and dimethoxybenzene that is commonly found as a plant metabolite.

In biochemistry, naturally occurring phenols are natural products containing at least one phenol functional group. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.

Tetsuo Nozoe was a Japanese organic chemist. He is known for the discovery of hinokitiol, a seven-membered aromatic compound, and studying non-benzenoid aromatic compounds.