
Pharmacology is a branch of medicine, biology and pharmaceutical sciences concerned with drug or medication action, where a drug may be defined as any artificial, natural, or endogenous molecule which exerts a biochemical or physiological effect on the cell, tissue, organ, or organism. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function. If substances have medicinal properties, they are considered pharmaceuticals.

Intravenous therapy is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutrients for those who cannot, or will not—due to reduced mental states or otherwise—consume food or water by mouth. It may also be used to administer medications or other medical therapy such as blood products or electrolytes to correct electrolyte imbalances. Attempts at providing intravenous therapy have been recorded as early as the 1400s, but the practice did not become widespread until the 1900s after the development of techniques for safe, effective use.
Therapeutic drug monitoring (TDM) is a branch of clinical chemistry and clinical pharmacology that specializes in the measurement of medication levels in blood. Its main focus is on drugs with a narrow therapeutic range, i.e. drugs that can easily be under- or overdosed. TDM aimed at improving patient care by individually adjusting the dose of drugs for which clinical experience or clinical trials have shown it improved outcome in the general or special populations. It can be based on a a priori pharmacogenetic, demographic and clinical information, and/or on the a posteriori measurement of blood concentrations of drugs or biological surrogate or end-point markers of effect.
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.

ADME is an abbreviation in pharmacokinetics and pharmacology for "absorption, distribution, metabolism, and excretion", and describes the disposition of a pharmaceutical compound within an organism. The four criteria all influence the drug levels and kinetics of drug exposure to the tissues and hence influence the performance and pharmacological activity of the compound as a drug. Sometimes, liberation and/or toxicity are also considered, yielding LADME, ADMET, or LADMET.

Bioequivalence is a term in pharmacokinetics used to assess the expected in vivo biological equivalence of two proprietary preparations of a drug. If two products are said to be bioequivalent it means that they would be expected to be, for all intents and purposes, the same.

Physiologically based pharmacokinetic (PBPK) modeling is a mathematical modeling technique for predicting the absorption, distribution, metabolism and excretion (ADME) of synthetic or natural chemical substances in humans and other animal species. PBPK modeling is used in pharmaceutical research and drug development, and in health risk assessment for cosmetics or general chemicals.
Biological half-life of a biological substance such as medication is the time it takes from its maximum concentration (Cmax) to half of its maximum concentration in the blood plasma, and is denoted by the abbreviation .
In pharmacokinetics, a loading dose is an initial higher dose of a drug that may be given at the beginning of a course of treatment before dropping down to a lower maintenance dose.
Pharmacokinetics, sometimes abbreviated as PK, is a branch of pharmacology dedicated to determine the fate of substances administered to a living organism. The substances of interest include any chemical xenobiotic such as: pharmaceutical drugs, pesticides, food additives, cosmetics, etc. It attempts to analyze chemical metabolism and to discover the fate of a chemical from the moment that it is administered up to the point at which it is completely eliminated from the body. Pharmacokinetics is the study of how an organism affects a drug, whereas pharmacodynamics (PD) is the study of how the drug affects the organism. Both together influence dosing, benefit, and adverse effects, as seen in PK/PD models.
A dose is a measured quantity of a medicine, nutrient, or pathogen which is delivered as a unit. The greater the quantity delivered, the larger the dose. Doses are most commonly measured for compounds in medicine. The term is usually applied to the quantity of a drug or other agent administered for therapeutic purposes, but may be used to describe any case where a substance is introduced to the body. In nutrition, the term is usually applied to how much of a specific nutrient is in a person's diet or in a particular food, meal, or dietary supplement. For bacterial or viral agents, dose typically refers to the amount of the pathogen required to infect a host. For information on dosage of toxic substances, see Toxicology. For information on excessive intake of pharmaceutical agents, see Drug overdose.

Deramciclane (EGIS-3886) is a non-benzodiazepine-type anxiolytic drug to treat various types of anxiety disorders. Deramciclane is a unique alternative to current anxiolytics on the market because it has a novel chemical structure and target. It acts as an antagonist at the 5-HT2A receptor, as an inverse agonist at the 5-HT2C receptor, and as a GABA reuptake inhibitor. The two serotonin receptors are G protein-coupled receptors and are two of the main excitatory serotonin receptor types. Their excitation has been implicated in anxiety and mood. Deramciclane does not affect CYP3A4 activity in metabolizing other drugs, but it is a weak inhibitor of CYP2D6. Some studies also show the drug to have moderate affinity to dopamine D2 receptors and low affinity to dopamine receptor D1. Researchers are looking for alternatives to benzodiazepines for anxiolytic use because benzodiazepine drugs have sedative and muscle relaxant side effects.
Cmin is a term used in pharmacokinetics for the minimum blood plasma concentration reached by a drug during the time interval between administration of two doses. This definition is slightly different from Ctrough, the concentration immediately prior to administration of the next dose. Cmin is the opposite of Cmax, the maximum concentration that the drug reaches. Cmin must be above certain thresholds, such as the minimum inhibitory concentration (MIC), to achieve a therapeutic effect.
Cmax is the maximum serum concentration that a drug achieves in a specified compartment or test area of the body after the drug has been administered and before the administration of a second dose. It is a standard measurement in pharmacokinetics.
In the field of pharmacokinetics, the area under the curve (AUC) is the definite integral of a curve that describes the variation of a drug concentration in blood plasma as a function of time. In practice, the drug concentration is measured at certain discrete points in time and the trapezoidal rule is used to estimate AUC.
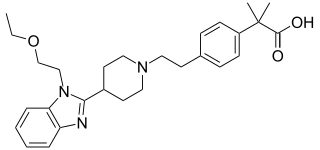
Bilastine, sold under the brand name Ilaxten among others, is a second-generation antihistamine medication which is used in the treatment of allergic rhinoconjunctivitis and urticaria (hives).

The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for safety in a few human subjects, then expand to many study participants to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays.
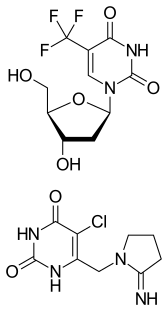
Trifluridine/tipiracil, sold under the brand name Lonsurf, is a fixed-dose combination medication that is used as a third- or fourth-line treatment of metastatic colorectal cancer or gastric cancer, after chemotherapy and targeted therapeutics have failed. It is a combination of two active pharmaceutical ingredients: trifluridine, a nucleoside analog, and tipiracil, a thymidine phosphorylase inhibitor. Tipiracil prevents rapid metabolism of trifluridine, increasing the bioavailability of trifluridine.

In pharmacology the elimination or excretion of a drug is understood to be any one of a number of processes by which a drug is eliminated from an organism either in an unaltered form or modified as a metabolite. The kidney is the main excretory organ although others exist such as the liver, the skin, the lungs or glandular structures, such as the salivary glands and the lacrimal glands. These organs or structures use specific routes to expel a drug from the body, these are termed elimination pathways:
Pertuzumab/trastuzumab/hyaluronidase, sold under the brand name Phesgo, is a fixed-dose combination medication to treat adults with HER2-positive breast cancer that has spread to other parts of the body, and for treatment of adults with early HER2-positive breast cancer. It contains pertuzumab, trastuzumab, and hyaluronidase–zzxf. It is injected under the skin via subcutaneous injection in the thigh. In the European Union, Phesgo contains the active ingredients pertuzumab and trastuzumab along with the enzyme vorhyaluronidase alfa.









