
A flavoring, also known as flavor or flavorant, is a food additive used to improve the taste or smell of food. It changes the perceptual impression of food as determined primarily by the chemoreceptors of the gustatory and olfactory systems. Along with additives, other components like sugars determine the taste of food.

Citrus is a genus of flowering trees and shrubs in the rue family, Rutaceae. Plants in the genus produce citrus fruits, including important crops such as oranges, lemons, grapefruits, pomelos, and limes. The genus Citrus is native to South Asia, East Asia, Southeast Asia, Melanesia, and Australia. Various citrus species have been used and domesticated by indigenous cultures in these areas since ancient times. From there its cultivation spread into Micronesia and Polynesia by the Austronesian expansion ; and to the Middle East and the Mediterranean via the incense trade route, and onwards to Europe and the Americas.

The grapefruit is a subtropical citrus tree known for its relatively large, sour to semi-sweet, somewhat bitter fruit. The interior flesh is segmented and varies in color from pale yellow to dark pink/red.

A clementine is a tangor, a citrus fruit hybrid between a willowleaf mandarin orange and a sweet orange, named in honor of Clément Rodier, a French missionary who first discovered and propagated the cultivar in Algeria. The exterior is a deep orange colour with a smooth, glossy appearance. Clementines can be separated into 7 to 14 segments. Similar to tangerines, they tend to be easy to peel. They are typically juicy and sweet, with less acid than oranges. Their oils, like other citrus fruits, contain mostly limonene as well as myrcene, linalool, α-pinene and many complex aromatics.

Yuzu is a citrus fruit and plant in the family Rutaceae of East Asian origin. Yuzu has been cultivated mainly in East Asia, though recently also in New Zealand, Australia, Spain, Italy, and France.

An aroma compound, also known as an odorant, aroma, fragrance or flavoring, is a chemical compound that has a smell or odor. For an individual chemical or class of chemical compounds to impart a smell or fragrance, it must be sufficiently volatile for transmission via the air to the olfactory system in the upper part of the nose. As examples, various fragrant fruits have diverse aroma compounds, particularly strawberries which are commercially cultivated to have appealing aromas, and contain several hundred aroma compounds.

Linalool refers to two enantiomers of a naturally occurring terpene alcohol found in many flowers and spice plants. Linalool has multiple commercial applications, the majority of which are based on its pleasant scent. A colorless oil, linalool is classified as an acyclic monoterpenoid. In plants, it is a metabolite, a volatile oil component, an antimicrobial agent, and an aroma compound. Linalool has uses in manufacturing of soaps, fragrances, food additives as flavors, household products, and insecticides. Esters of linalool are referred to as linalyl, e.g. linalyl pyrophosphate, an isomer of geranyl pyrophosphate.

Limonene is a colorless liquid aliphatic hydrocarbon classified as a cyclic monoterpene, and is the major component in the volatile oil of citrus fruit peels. The (+)-isomer, occurring more commonly in nature as the fragrance of oranges, is a flavoring agent in food manufacturing. It is also used in chemical synthesis as a precursor to carvone and as a renewables-based solvent in cleaning products. The less common (-)-isomer has a piny, turpentine-like odor, and is found in the edible parts of such plants as caraway, dill, and bergamot orange plants.
The odor detection threshold is the lowest concentration of a certain odor compound that is perceivable by the human sense of smell. The threshold of a chemical compound is determined in part by its shape, polarity, partial charges, and molecular mass. The olfactory mechanisms responsible for a compound's different detection threshold is not well understood. As such, odor thresholds cannot be accurately predicted. Rather, they must be measured through extensive tests using human subjects in laboratory settings.

Octyl acetate, or octyl ethanoate, is an organic compound with the formula CH3(CH2)7O2CCH3. It is classified as an ester that is formed from 1-octanol (octyl alcohol) and acetic acid. It is found in oranges, grapefruits, and other citrus products.
Grapefruit mercaptan is a natural organic compound found in grapefruit. It is a monoterpenoid that contains a thiol functional group. Structurally a hydroxy group of terpineol is replaced by the thiol in grapefruit mercaptan, so it also called thioterpineol. Volatile thiols typically have very strong, often unpleasant odors that can be detected by humans in very low concentrations. Grapefruit mercaptan has a very potent, but not unpleasant, odor, and it is the chemical constituent primarily responsible for the aroma of grapefruit. This characteristic aroma is a property of only the R enantiomer.

Zest is a food ingredient that is prepared by scraping or cutting from the rind of unwaxed citrus fruits such as lemon, orange, citron, and lime. Zest is used to add flavor to foods.

Nootkatone is an organic compound, a sesquiterpenoid, which means that it is a C15 derivative that also contains an oxygen-containing functional group. It is the most valuable aromatic compo of grapefruit. Nootkatone was originally isolated from the wood of the Alaskan yellow cedar, Cupressus nootkatensis. The species name, nootkatensis, is derived from the language of the Nuu-Chah-Nulth people of Canada.

Methyl anthranilate, also known as MA, methyl 2-aminobenzoate, or carbomethoxyaniline, is an ester of anthranilic acid. Its chemical formula is C8H9NO2. It has a strong and fruity grape smell, and one of its key uses is as a flavoring agent.
(E,E)-2,4-Decadienal is an aromatic substance found in butter, cooked beef, fish, potato chips, roasted peanut, buckwheat and wheat bread crumb. In an isolated state, it smells of deep fat flavor, characteristic of chicken aroma (at 10ppm). At lower concentration, it has the odor of citrus, orange or grapefruit. It might be carcinogenic. It has been used as aroma in the EU, but use restrictions apply until the required data have been submitted.
Valencene synthase is an enzyme with systematic name (2E,6E)-farnesyl-diphosphate diphosphate-lyase (valencene-forming). It is a terpene cyclase enzyme responsible for the biosynthesis of valencene, a sesquiterpene, using farnesyl pyrophosphate as its substrate. The first such enzyme was isolated using orange cDNA. This enzyme catalyses the following chemical reaction
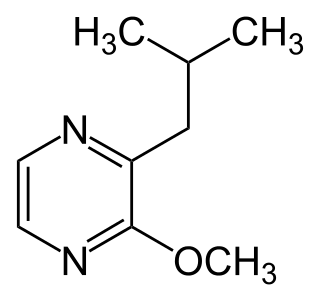
3-Isobutyl-2-methoxypyrazine is a methoxypyrazine that is very similar to isopropyl methoxy pyrazine except that the alkyl side-group contains an isobutyl group attached to the carbon alpha to the methoxy sidegroup instead of an isopropyl side-group at that same carbon position.
This article covers protein engineering of cytochrome (CYP) P450 enzymes. P450s are involved in a range of biochemical catabolic and anabolic process. Natural P450s can perform several different types of chemical reactions including hydroxylations, N,O,S-dealkylations, epoxidations, sulfoxidations, aryl-aryl couplings, ring contractions and expansions, oxidative cyclizations, alcohol/aldehyde oxidations, desaturations, nitrogen oxidations, decarboxylations, nitrations, as well as oxidative and reductive dehalogenations. Engineering efforts often strive for 1) improved stability 2) improved activity 3) improved substrate scope 4) enabled ability to catalyze unnatural reactions. P450 engineering is an emerging field in the areas of chemical biology and synthetic organic chemistry (chemoenzymatic).

Kawachi Bankan, also called Mishokan and Uwa Gold, is a Citrus hybrid cultivated for its edible fruit.














