Applications

Therapeutic and imaging agents
VLPs are a candidate delivery system for genes or other therapeutics. [12] These drug delivery agents have been shown to effectively target cancer cells in vitro. [13] It is hypothesized that VLPs may accumulate in tumor sites due to the enhanced permeability and retention effect, which could be useful for drug delivery or tumor imaging. [14]
Vaccines
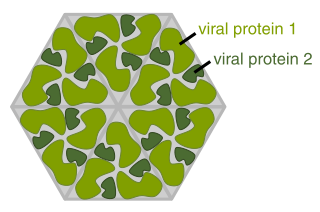
VLPs are useful as vaccines. VLPs contain repetitive, high density displays of viral surface proteins that present conformational viral epitopes that can elicit strong T cell and B cell immune responses. [15] The particles' small radius of roughly 20-200 nm allows sufficient draining into lymph nodes. Since VLPs cannot replicate, they provide a safer alternative to attenuated viruses. VLPs were used to develop FDA-approved vaccines for Hepatitis B and human papillomavirus, which are commercially available. [16]
A selection of viruslike particle-based vaccines against human papilloma virus (HPV) such as Cervarix by GlaxoSmithKline along with Gardasil and Gardasil-9, are available, produced by Merck & Co. Gardasil consists of recombinant VLPs assembled from the L1 proteins of HPV types 6, 11, 16, and 18 expressed in yeast. It is adjuvanted with aluminum hydroxyphosphate sulfate. Gardasil-9 consists of L1 epitopes of 31, 33, 45, 52 and 58 in addition to the listed L1 epitopes found in Gardasil. Cervarix consists of recombinant VLPs assembled from the L1 proteins of HPV types 16 and 18, expressed in insect cells, and is adjuvanted with 3-O-Desacyl-4-monophosphoryl lipid (MPL) A and aluminum hydroxide. [17]
The first VLP vaccine that addresses malaria, Mosquirix, (RTS,S) has been approved by EU regulators. It was expressed in yeast. RTS,S is a portion of the Plasmodium falciparum circumsporozoite protein fused to the Hepatitis B surface antigen (RTS), combined with Hepatitis B surface antigen (S), and adjuvanted with AS01 (consisting of (MPL)A and saponin).
Vaccine production can begin as soon as the virus strain is sequenced and can take as little as 12 weeks, compared to 9 months for traditional vaccines. In early clinical trials, VLP vaccines for influenza appeared to provide complete protection against both the Influenza A virus subtype H5N1 and the 1918 flu pandemic. [18] Novavax and Medicago Inc. have run clinical trials of their VLP flu vaccines. [19] [20] Several VLP vaccines for COVID-19, including Novavax, are under development. [16] [21]
VLPs have been used to develop a pre-clinical vaccine candidate against chikungunya virus. [15]
Bio-inspired Material Synthesis
Compartmentalization is a common theme in biology. Nature is full of examples of hierarchically compartmentalized multicomponent structures that self-assembles from individual building blocks. Taking inspiration from nature, synthetic approaches using polymers, phase-separated microdroplets, lipids and proteins have been used to mimic hierarchical compartmentalization of natural systems and to form functional bio-inspired nanomaterials. [22] [23] [24] For example, protein self-assembly was used to encapsulate multiple copies of ferritin protein cages as sub-compartments inside P22 virus-like particle as larger compartment essentially forming a Matryoshka-like nested cage-within-cage structure. [25] The authors further demonstrated stoichiometric encapsulation of cellobiose-hydrolysing β-glycosidase enzyme CelB along with ferritin protein cages using in-vitro self-assembly strategy to form multi-compartment cell-inspired protein cage structure. Using similar strategy, glutathione biosynthesizing enzymes were encapsulated inside bacteriophage P22 virus-like particles. [26] In a separate research, 3.5 nm small Cytochrome C with peroxidase-like activity was encapsulated inside a 9 nm small Dps protein cage to form organelle-inspired protein cage structure. [27]
Lipoparticle technology
The VLP lipoparticle was developed to aid the study of integral membrane proteins. [28] Lipoparticles are stable, highly purified, homogeneous VLPs that are engineered to contain high concentrations of a conformationally intact membrane protein of interest. Integral Membrane proteins are involved in diverse biological functions and are targeted by nearly 50% of existing therapeutic drugs. However, because of their hydrophobic domains, membrane proteins are difficult to manipulate outside of living cells. Lipoparticles can incorporate a wide variety of structurally intact membrane proteins, including G protein-coupled receptors (GPCR)s, ion channels and viral Envelopes. Lipoparticles provide a platform for numerous applications including antibody screening, production of immunogens and ligand binding assays. [29] [30]