
Vitamin K is a family of structurally similar, fat-soluble vitamers found in foods and marketed as dietary supplements. The human body requires vitamin K for post-synthesis modification of certain proteins that are required for blood coagulation or for controlling binding of calcium in bones and other tissues. The complete synthesis involves final modification of these so-called "Gla proteins" by the enzyme gamma-glutamyl carboxylase that uses vitamin K as a cofactor.

An anticoagulant, commonly known as a blood thinner, is a chemical substance that prevents or reduces the coagulation of blood, prolonging the clotting time. Some occur naturally in blood-eating animals, such as leeches and mosquitoes, which help keep the bite area unclotted long enough for the animal to obtain blood.

Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The process of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.

Warfarin is an anticoagulant used as a medication under several brand names including Coumadin. While the drug is described as a "blood thinner", it does not reduce viscosity but rather inhibits coagulation. Accordingly, it is commonly used to prevent blood clots in the circulatory system such as deep vein thrombosis and pulmonary embolism, and to protect against stroke in people who have atrial fibrillation, valvular heart disease, or artificial heart valves. Less commonly, it is used following ST-segment elevation myocardial infarction (STEMI) and orthopedic surgery. It is usually taken by mouth, but may also be administered intravenously.
Low-molecular-weight heparin (LMWH) is a class of anticoagulant medications. They are used in the prevention of blood clots and, in the treatment of venous thromboembolism, and the treatment of myocardial infarction.

The prothrombin time (PT) – along with its derived measures of prothrombin ratio (PR) and international normalized ratio (INR) – is an assay for evaluating the extrinsic pathway and common pathway of coagulation. This blood test is also called protime INR and PT/INR. They are used to determine the clotting tendency of blood, in such things as the measure of warfarin dosage, liver damage, and vitamin K status. PT measures the following coagulation factors: I (fibrinogen), II (prothrombin), V (proaccelerin), VII (proconvertin), and X.
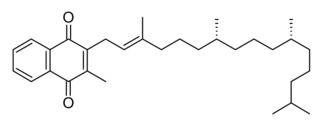
Phytomenadione, also known as vitamin K1 or phylloquinone, is a vitamin found in food and used as a dietary supplement. It is on the World Health Organization's List of Essential Medicines.

Hypoprothrombinemia is a rare blood disorder in which a deficiency in immunoreactive prothrombin, produced in the liver, results in an impaired blood clotting reaction, leading to an increased physiological risk for spontaneous bleeding. This condition can be observed in the gastrointestinal system, cranial vault, and superficial integumentary system, affecting both the male and female population. Prothrombin is a critical protein that is involved in the process of hemostasis, as well as illustrating procoagulant activities. This condition is characterized as an autosomal recessive inheritance congenital coagulation disorder affecting 1 per 2,000,000 of the population, worldwide, but is also attributed as acquired.

Rivaroxaban, sold under the brand name Xarelto among others, is an anticoagulant medication used to treat and prevent blood clots. Specifically it is used to treat deep vein thrombosis and pulmonary emboli and prevent blood clots in atrial fibrillation and following hip or knee surgery. It is taken by mouth.

Warfarin-induced skin necrosis is a condition in which skin and subcutaneous tissue necrosis occurs due to acquired protein C deficiency following treatment with anti-vitamin K anticoagulants.

Protein C deficiency is a rare genetic trait that predisposes to thrombotic disease. It was first described in 1981. The disease belongs to a group of genetic disorders known as thrombophilias. Protein C deficiency is associated with an increased incidence of venous thromboembolism, whereas no association with arterial thrombotic disease has been found.

Phenprocoumon is a long-acting blood thinner drug to be taken by mouth, and a coumarin derivative. It acts as a vitamin K antagonist and inhibits blood clotting (coagulation) by blocking synthesis of coagulation factors II, VII, IX and X. It is used for the prophylaxis and treatment of thromboembolic disorders such as heart attacks and pulmonary (lung) embolism. The most common adverse effect is bleeding. The drug interacts with a large number of other medications, including aspirin and St John's Wort. It is the standard coumarin used in Germany, Austria, and other European countries.

Brodifacoum is a highly lethal 4-hydroxycoumarin vitamin K antagonist anticoagulant poison. In recent years, it has become one of the world's most widely used pesticides. It is typically used as a rodenticide, but is also used to control larger pests such as possums.

Vitamin K deficiency bleeding (VKDB) of the newborn, previously known as haemorrhagic disease of the newborn, is a rare form of bleeding disorder that affects newborns and young infants due to low stores of vitamin K at birth. It commonly presents with intracranial haemorrhage with the risk of brain damage or death.
Prothrombin complex concentrate (PCC), also known as factor IX complex, sold under the brand name Kcentra among others, is a combination medication made up of blood clotting factors II, IX, and X. Some versions also contain factor VII. It is used to treat and prevent bleeding in hemophilia B if pure factor IX is not available. It may also be used for reversal of warfarin therapy. It is given by slow injection into a vein.
Purpura fulminans is an acute, often fatal, thrombotic disorder which manifests as blood spots, bruising and discolouration of the skin resulting from coagulation in small blood vessels within the skin and rapidly leads to skin necrosis and disseminated intravascular coagulation.

The human gene VKORC1 encodes for the enzyme, Vitamin K epOxide Reductase Complex (VKORC) subunit 1. This enzymatic protein complex is responsible for reducing vitamin K 2,3-epoxide to its active form, which is important for effective clotting (coagulation). In humans, mutations in this gene can be associated with deficiencies in vitamin-K-dependent clotting factors.
Direct factor Xa inhibitors (xabans) are anticoagulants, used to both treat and prevent blood clots in veins, and prevent stroke and embolism in people with atrial fibrillation (AF).
Vitamin K deficiency results from insufficient dietary vitamin K1 or vitamin K2 or both.
Direct thrombin inhibitors (DTIs) are a class of anticoagulant drugs that can be used to prevent and treat embolisms and blood clots caused by various diseases. They inhibit thrombin, a serine protease which affects the coagulation cascade in many ways. DTIs have undergone rapid development since the 90's. With technological advances in genetic engineering the production of recombinant hirudin was made possible which opened the door to this new group of drugs. Before the use of DTIs the therapy and prophylaxis for anticoagulation had stayed the same for over 50 years with the use of heparin derivatives and warfarin which have some well known disadvantages. DTIs are still under development, but the research focus has shifted towards factor Xa inhibitors, or even dual thrombin and fXa inhibitors that have a broader mechanism of action by both inhibiting factor IIa (thrombin) and Xa. A recent review of patents and literature on thrombin inhibitors has demonstrated that the development of allosteric and multi-mechanism inhibitors might lead the way to a safer anticoagulant.














