
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of such infections. They may either kill or inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the ones which cause the common cold or influenza; drugs which inhibit growth of viruses are termed antiviral drugs or antivirals rather than antibiotics. They are also not effective against fungi; drugs which inhibit growth of fungi are called antifungal drugs.
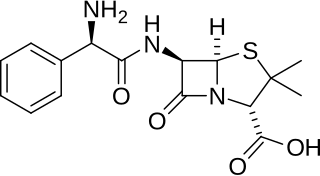
Ampicillin is an antibiotic belonging to the aminopenicillin class of the penicillin family. The drug is used to prevent and treat a number of bacterial infections, such as respiratory tract infections, urinary tract infections, meningitis, salmonellosis, and endocarditis. It may also be used to prevent group B streptococcal infection in newborns. It is used by mouth, by injection into a muscle, or intravenously.

Antimicrobial resistance (AMR) occurs when microbes evolve mechanisms that protect them from the effects of antimicrobials. All classes of microbes can evolve resistance where the drugs are no longer effective. Fungi evolve antifungal resistance, viruses evolve antiviral resistance, protozoa evolve antiprotozoal resistance, and bacteria evolve antibiotic resistance. Together all of these come under the umbrella of antimicrobial resistance. Microbes resistant to multiple antimicrobials are called multidrug resistant (MDR) and are sometimes referred to as superbugs. Although antimicrobial resistance is a naturally occurring process, it is often the result of improper usage of the drugs and management of the infections.
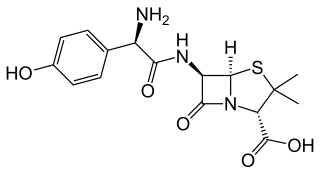
Amoxicillin is an antibiotic medication belonging to the aminopenicillin class of the penicillin family. The drug is used to treat bacterial infections such as middle ear infection, strep throat, pneumonia, skin infections, odontogenic infections, and urinary tract infections. It is taken by mouth, or less commonly by injection.

A broad-spectrum antibiotic is an antibiotic that acts on the two major bacterial groups, Gram-positive and Gram-negative, or any antibiotic that acts against a wide range of disease-causing bacteria. These medications are used when a bacterial infection is suspected but the group of bacteria is unknown or when infection with multiple groups of bacteria is suspected. This is in contrast to a narrow-spectrum antibiotic, which is effective against only a specific group of bacteria. Although powerful, broad-spectrum antibiotics pose specific risks, particularly the disruption of native, normal bacteria and the development of antimicrobial resistance. An example of a commonly used broad-spectrum antibiotic is ampicillin.

Clindamycin is a lincosamide antibiotic medication used for the treatment of a number of bacterial infections, including osteomyelitis (bone) or joint infections, pelvic inflammatory disease, strep throat, pneumonia, acute otitis media, and endocarditis. It can also be used to treat acne, and some cases of methicillin-resistant Staphylococcus aureus (MRSA). In combination with quinine, it can be used to treat malaria. It is available by mouth, by injection into a vein, and as a cream or a gel to be applied to the skin or in the vagina.

Amoxicillin/clavulanic acid, also known as co-amoxiclav or amox-clav, sold under the brand name Augmentin, among others, is an antibiotic medication used for the treatment of a number of bacterial infections. It is a combination consisting of amoxicillin, a β-lactam antibiotic, and potassium clavulanate, a β-lactamase inhibitor. It is specifically used for otitis media, streptococcal pharyngitis, pneumonia, cellulitis, urinary tract infections, and animal bites. It is taken by mouth or by injection into a vein.

Tigecycline, sold under the brand name Tygacil, is a tetracycline antibiotic medication for a number of bacterial infections. It is a glycylcycline class drug that is administered intravenously. It was developed in response to the growing rate of antibiotic resistant bacteria such as Staphylococcus aureus, Acinetobacter baumannii, and E. coli. As a tetracycline derivative antibiotic, its structural modifications has expanded its therapeutic activity to include Gram-positive and Gram-negative organisms, including those of multi-drug resistance.

Cefotaxime is an antibiotic used to treat a number of bacterial infections in human, other animals and plant tissue culture. Specifically in humans it is used to treat joint infections, pelvic inflammatory disease, meningitis, pneumonia, urinary tract infections, sepsis, gonorrhea, and cellulitis. It is given either by injection into a vein or muscle.

Fosfomycin, sold under the brand name Monurol among others, is an antibiotic primarily used to treat lower urinary tract infections. It is not indicated for kidney infections. Occasionally it is used for prostate infections. It is generally taken by mouth.

Antibiotic misuse, sometimes called antibiotic abuse or antibiotic overuse, refers to the misuse or overuse of antibiotics, with potentially serious effects on health. It is a contributing factor to the development of antibiotic resistance, including the creation of multidrug-resistant bacteria, informally called "super bugs": relatively harmless bacteria can develop resistance to multiple antibiotics and cause life-threatening infections.

Antibiotic use in livestock is the use of antibiotics for any purpose in the husbandry of livestock, which includes treatment when ill (therapeutic), treatment of a group of animals when at least one is diagnosed with clinical infection (metaphylaxis), and preventative treatment (prophylaxis). Antibiotics are an important tool to treat animal as well as human disease, safeguard animal health and welfare, and support food safety. However, used irresponsibly, this may lead to antibiotic resistance which may impact human, animal and environmental health.
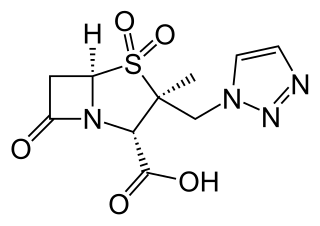
Ceftolozane/tazobactam, sold under the brand name Zerbaxa, is a fixed-dose combination antibiotic medication used for the treatment of complicated urinary tract infections and complicated intra-abdominal infections in adults. Ceftolozane is a cephalosporin antibiotic, developed for the treatment of infections with gram-negative bacteria that are resistant to conventional antibiotics. It was studied for urinary tract infections, intra-abdominal infections and ventilator-associated bacterial pneumonia.
Antimicrobial stewardship (AMS) refers to coordinated efforts to promote the optimal use of antimicrobial agents, including drug choice, dosing, route, and duration of administration.
There are many circumstances during dental treatment where antibiotics are prescribed by dentists to prevent further infection. The most common antibiotic prescribed by dental practitioners is penicillin in the form of amoxicillin, however many patients are hypersensitive to this particular antibiotic. Therefore, in the cases of allergies, erythromycin is used instead.
Antimicrobial resistance (AMR) directly kills about 1,600 people each year in Australia. This is a currently serious threat to both humans and animals in the country. Antimicrobial resistance occurs when a microorganism evolves and gains the ability to become more resistant or completely resistant to the medicine that was previously used to treat it. Drug-resistant bacteria are increasingly difficult to treat, requiring replacement or higher-dose drugs that may be more expensive or more toxic. Resistance can develop through one of the three mechanisms: natural resistant ability in some types of microorganisms, a mutation in genes or receiving the resistance from another species. Antibodies appear naturally due to random mutations, or more often after gradual accumulation over time, and because of abuse of antibiotics. Multidrug-resistance, or MDR, are the microorganisms that are resistant to many types of antimicrobials. "Superbugs" is the term also used for multidrug-resistant microbes, or totally drug-resistant (TDR).
The Society of Infectious Diseases Pharmacists (SIDP) is a non-profit association of pharmacists and other allied health professionals who specialize in infectious diseases and antimicrobial stewardship. According to the Board of Pharmaceutical Specialties, clinical pharmacists specializing in infectious diseases are trained in the use of microbiology and pharmacology to develop, implement, and monitor drug regimens that incorporate the pharmacodynamics and pharmacokinetics of antimicrobials for patients.
Alison Helen Holmes is a British infectious diseases specialist, who is a professor at Imperial College London and the University of Liverpool. Holmes serves as Director of the National Institute for Health Research (NIHR) Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance and Consultant at Hammersmith Hospital. Holmes is on the Executive Committee of the International Society of Infectious Diseases, and she serves on a variety of World Health Organization (WHO) expert groups related to antimicrobial use, Antimicrobial Resistance (AMR), infection prevention and sepsis. Her research considers how to mitigate antimicrobial resistance.













