
Ciprofloxacin is a fluoroquinolone antibiotic used to treat a number of bacterial infections. This includes bone and joint infections, intra-abdominal infections, certain types of infectious diarrhea, respiratory tract infections, skin infections, typhoid fever, and urinary tract infections, among others. For some infections it is used in addition to other antibiotics. It can be taken by mouth, as eye drops, as ear drops, or intravenously.
A urinary tract infection (UTI) is an infection that affects a part of the urinary tract. Lower urinary tract infections may involve the bladder (cystitis) or urethra (urethritis) while upper urinary tract infections affect the kidney (pyelonephritis). Symptoms from a lower urinary tract infection include suprapubic pain, painful urination (dysuria), frequency and urgency of urination despite having an empty bladder. Symptoms of a kidney infection, on the other hand, are more systemic and include fever or flank pain usually in addition to the symptoms of a lower UTI. Rarely, the urine may appear bloody. Symptoms may be vague or non-specific at the extremities of age.

Trimethoprim (TMP) is an antibiotic used mainly in the treatment of bladder infections. Other uses include for middle ear infections and travelers' diarrhea. With sulfamethoxazole or dapsone it may be used for Pneumocystis pneumonia in people with HIV/AIDS. It is taken orally.

Levofloxacin, sold under the brand name Levaquin among others, is an antibiotic medication. It is used to treat a number of bacterial infections including acute bacterial sinusitis, pneumonia, H. pylori, urinary tract infections, Legionnaires' disease, chronic bacterial prostatitis, and some types of gastroenteritis. Along with other antibiotics it may be used to treat tuberculosis, meningitis, or pelvic inflammatory disease. Use is generally recommended only when other options are not available. It is available by mouth, intravenously, and in eye drop form.

Ofloxacin is a quinolone antibiotic useful for the treatment of a number of bacterial infections. When taken by mouth or injection into a vein, these include pneumonia, cellulitis, urinary tract infections, prostatitis, plague, and certain types of infectious diarrhea. Other uses, along with other medications, include treating multidrug resistant tuberculosis. An eye drop may be used for a superficial bacterial infection of the eye and an ear drop may be used for otitis media when a hole in the ear drum is present.

Cefixime, sold under the brand name Suprax among others, is an antibiotic medication used to treat a number of bacterial infections. These infections include otitis media, strep throat, pneumonia, urinary tract infections, gonorrhea, and Lyme disease. For gonorrhea typically only one dose is required. In the United States it is a second-line treatment to ceftriaxone for gonorrhea. It is taken by mouth.

Pyelonephritis is inflammation of the kidney, typically due to a bacterial infection. Symptoms most often include fever and flank tenderness. Other symptoms may include nausea, burning with urination, and frequent urination. Complications may include pus around the kidney, sepsis, or kidney failure.

Cefaclor, sold under the trade name Ceclor among others, is a second-generation cephalosporin antibiotic used to treat certain bacterial infections such as pneumonia and infections of the ear, lung, skin, throat, and urinary tract. It is also available from other manufacturers as a generic.
Staphylococcus saprophyticus is a Gram-positive coccus belonging to the genus Staphylococcus. S. saprophyticus is a common cause of community-acquired urinary tract infections.

Tobramycin is an aminoglycoside antibiotic derived from Streptomyces tenebrarius that is used to treat various types of bacterial infections, particularly Gram-negative infections. It is especially effective against species of Pseudomonas.

Norfloxacin, sold under the brand name Noroxin among others, is an antibiotic that belongs to the class of fluoroquinolone antibiotics. It is used to treat urinary tract infections, gynecological infections, inflammation of the prostate gland, gonorrhea and bladder infection. Eye drops were approved for use in children older than one year of age.

Nitrofurans are a class of drugs typically used as antibiotics or antimicrobials. The defining structural component is a furan ring with a nitro group.

Cefditoren, also known as cefditoren pivoxil is an antibiotic used to treat infections caused by Gram-positive and Gram-negative bacteria that are resistant to other antibiotics. It is mainly used for treatment of community acquired pneumonia. It is taken by mouth and is in the cephalosporin family of antibiotics, which is part of the broader beta-lactam group of antibiotics.

Fosfomycin, sold under the brand name Monurol among others, is an antibiotic primarily used to treat lower urinary tract infections. It is not indicated for kidney infections. Occasionally it is used for prostate infections. It is generally taken by mouth.

Bacteriuria is the presence of bacteria in urine. Bacteriuria accompanied by symptoms is a urinary tract infection while that without is known as asymptomatic bacteriuria. Diagnosis is by urinalysis or urine culture. Escherichia coli is the most common bacterium found. People without symptoms should generally not be tested for the condition. Differential diagnosis include contamination.

Prulifloxacin is an older synthetic antibiotic of the fluoroquinolone class undergoing clinical trials prior to a possible NDA submission to the U.S. Food and Drug Administration (FDA). It is a prodrug which is metabolized in the body to the active compound ulifloxacin. It was developed over two decades ago by Nippon Shinyaku Co. and was patented in Japan in 1987 and in the United States in 1989.

Pivmecillinam (INN), or amdinocillin pivoxil (USAN), sold under the brand name Selexid and Pivya among others, is an orally active prodrug of mecillinam, an extended-spectrum penicillin antibiotic. Pivmecillinam is the pivaloyloxymethyl ester of mecillinam.
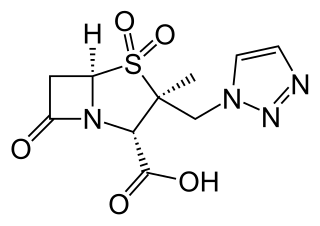
Ceftolozane/tazobactam, sold under the brand name Zerbaxa, is a fixed-dose combination antibiotic medication used for the treatment of complicated urinary tract infections and complicated intra-abdominal infections in adults. Ceftolozane is a cephalosporin antibiotic, developed for the treatment of infections with gram-negative bacteria that are resistant to conventional antibiotics. It was studied for urinary tract infections, intra-abdominal infections and ventilator-associated bacterial pneumonia.

Gepotidacin (INN) is an experimental antibiotic that acts as a topoisomerase type II inhibitor. It is being studied for the treatment of uncomplicated urinary tract infection and infection with Neisseria gonorrhoeae (gonorrhea), including multidrug resistant strains.
Meropenem/vaborbactam, sold under the brand name Vabomere among others, is a combination medication used to treat complicated urinary tract infections, complicated abdominal infections, and hospital-acquired pneumonia. It contains meropenem, a β-lactam antibiotic, and vaborbactam, a β-lactamase inhibitor. It is given by injection into a vein.





















