Ciprofloxacin is a fluoroquinolone antibiotic used to treat a number of bacterial infections. This includes bone and joint infections, intra-abdominal infections, certain types of infectious diarrhea, respiratory tract infections, skin infections, typhoid fever, and urinary tract infections, among others. For some infections it is used in addition to other antibiotics. It can be taken by mouth, as eye drops, as ear drops, or intravenously.

Levofloxacin, sold under the brand name Levaquin among others, is an antibiotic medication. It is used to treat a number of bacterial infections including acute bacterial sinusitis, pneumonia, H. pylori, urinary tract infections, chronic prostatitis, and some types of gastroenteritis. Along with other antibiotics it may be used to treat tuberculosis, meningitis, or pelvic inflammatory disease. Use is generally recommended only when other options are not available. It is available by mouth, intravenously, and in eye drop form.

Ofloxacin is a quinolone antibiotic useful for the treatment of a number of bacterial infections. When taken by mouth or injection into a vein, these include pneumonia, cellulitis, urinary tract infections, prostatitis, plague, and certain types of infectious diarrhea. Other uses, along with other medications, include treating multidrug resistant tuberculosis. An eye drop may be used for a superficial bacterial infection of the eye and an ear drop may be used for otitis media when a hole in the ear drum is present.
Nalidixic acid is the first of the synthetic quinolone antibiotics.

Norfloxacin, sold under the brand name Noroxin among others, is an antibiotic that belongs to the class of fluoroquinolone antibiotics. It is used to treat urinary tract infections, gynecological infections, inflammation of the prostate gland, gonorrhea and bladder infection. Eye drops were approved for use in children older than one year of age.

Trovafloxacin is a broad spectrum antibiotic that inhibits the uncoiling of supercoiled DNA in various bacteria by blocking the activity of DNA gyrase and topoisomerase IV. It was withdrawn from the market due to the risk of hepatotoxicity. It had better Gram-positive bacterial coverage but less Gram-negative coverage than the previous fluoroquinolones.

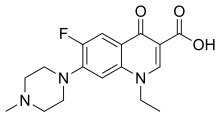
Moxifloxacin is an antibiotic, used to treat bacterial infections, including pneumonia, conjunctivitis, endocarditis, tuberculosis, and sinusitis. It can be given by mouth, by injection into a vein, and as an eye drop.
Enoxacin is an oral broad-spectrum fluoroquinolone antibacterial agent used in the treatment of urinary tract infections and gonorrhea. Insomnia is a common adverse effect. It is no longer available in the United States.

Sparfloxacin is a fluoroquinolone antibiotic used in the treatment of bacterial infections. It has a controversial safety profile.

Cinoxacin is a quinolone antibiotic that has been discontinued in the U.K. as well the United States, both as a branded drug or a generic. The marketing authorization of cinoxacin has been suspended throughout the EU.
Topoisomerase inhibitors are chemical compounds that block the action of topoisomerases, which are broken into two broad subtypes: type I topoisomerases (TopI) and type II topoisomerases (TopII). Topoisomerase plays important roles in cellular reproduction and DNA organization, as they mediate the cleavage of single and double stranded DNA to relax supercoils, untangle catenanes, and condense chromosomes in eukaryotic cells. Topoisomerase inhibitors influence these essential cellular processes. Some topoisomerase inhibitors prevent topoisomerases from performing DNA strand breaks while others, deemed topoisomerase poisons, associate with topoisomerase-DNA complexes and prevent the re-ligation step of the topoisomerase mechanism. These topoisomerase-DNA-inhibitor complexes are cytotoxic agents, as the un-repaired single- and double stranded DNA breaks they cause can lead to apoptosis and cell death. Because of this ability to induce apoptosis, topoisomerase inhibitors have gained interest as therapeutics against infectious and cancerous cells.
Fleroxacin is a quinolone antibiotic. It is sold under the brand names Quinodis and Megalocin.

Flumequine is a synthetic fluoroquinolone antibiotic used to treat bacterial infections. It is a first-generation fluoroquinolone antibacterial that has been removed from clinical use and is no longer being marketed. The marketing authorization of flumequine has been suspended throughout the EU. It kills bacteria by interfering with the enzymes that cause DNA to unwind and duplicate. Flumequine was used in veterinarian medicine for the treatment of enteric infections, as well as to treat cattle, swine, chickens, and fish, but only in a limited number of countries. It was occasionally used in France to treat urinary tract infections under the trade name Apurone. However this was a limited indication because only minimal serum levels were achieved.

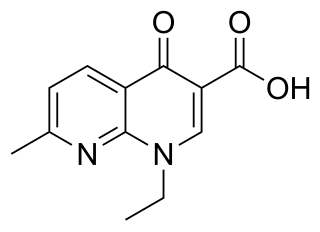
Prulifloxacin is an older synthetic antibiotic of the fluoroquinolone class undergoing clinical trials prior to a possible NDA submission to the U.S. Food and Drug Administration (FDA). It is a prodrug which is metabolized in the body to the active compound ulifloxacin. It was developed over two decades ago by Nippon Shinyaku Co. and was patented in Japan in 1987 and in the United States in 1989.

Nadifloxacin is a topical fluoroquinolone antibiotic for the treatment of acne vulgaris. It is also used to treat bacterial skin infections.
Difloxacin (INN), marketed under the trade name Dicural, is a second-generation, synthetic fluoroquinolone antibiotic used in veterinary medicine. It has broad-spectrum, concentration dependent, bactericidal activity; however, its efficacy is not as good as enrofloxacin or pradofloxacin.

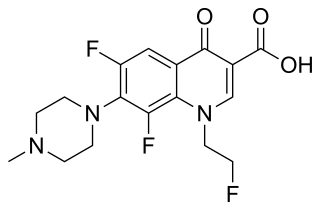
Pradofloxacin, sold under the brand name Veraflox, is a third-generation enhanced spectrum veterinary antibiotic of the fluoroquinolone class. It was developed by Bayer HealthCare AG, Animal Health GmbH, and received approval from the European Commission in April 2011 for prescription-only use in veterinary medicine for the treatment of bacterial infections in dogs and cats.

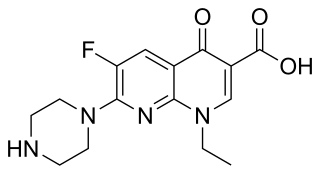
Clinafloxacin is an investigational fluoroquinolone antibiotic. Despite its promising antibiotic activity, the clinical development of clinafloxacin has been hampered by its risk for inducing serious side effects.

Quinolone antibiotics constitute a large group of broad-spectrum bacteriocidals that share a bicyclic core structure related to the substance 4-quinolone. They are used in human and veterinary medicine to treat bacterial infections, as well as in animal husbandry, specifically poultry production.

Neisseria gonorrhoeae, the bacterium that causes the sexually transmitted infection gonorrhea, has developed antibiotic resistance to many antibiotics. The bacteria was first identified in 1879.