
Aztreonam, sold under the brand name Azactam among others, is an antibiotic used primarily to treat infections caused by gram-negative bacteria such as Pseudomonas aeruginosa. This may include bone infections, endometritis, intra abdominal infections, pneumonia, urinary tract infections, and sepsis. It is given by intravenous or intramuscular injection or by inhalation.

Ofloxacin is a quinolone antibiotic useful for the treatment of a number of bacterial infections. When taken by mouth or injection into a vein, these include pneumonia, cellulitis, urinary tract infections, prostatitis, plague, and certain types of infectious diarrhea. Other uses, along with other medications, include treating multidrug resistant tuberculosis. An eye drop may be used for a superficial bacterial infection of the eye and an ear drop may be used for otitis media when a hole in the ear drum is present.

Carbenicillin is a bactericidal antibiotic belonging to the carboxypenicillin subgroup of the penicillins. It was discovered by scientists at Beecham and marketed as Pyopen. It has Gram-negative coverage which includes Pseudomonas aeruginosa but limited Gram-positive coverage. The carboxypenicillins are susceptible to degradation by beta-lactamase enzymes, although they are more resistant than ampicillin to degradation. Carbenicillin is also more stable at lower pH than ampicillin.

Ceftazidime, sold under the brand name Fortaz among others, is a third-generation cephalosporin antibiotic useful for the treatment of a number of bacterial infections. Specifically it is used for joint infections, meningitis, pneumonia, sepsis, urinary tract infections, malignant otitis externa, Pseudomonas aeruginosa infection, and vibrio infection. It is given by injection into a vein, muscle, or eye.

Tigecycline, sold under the brand name Tygacil, is a tetracycline antibiotic medication for a number of bacterial infections. It is a glycylcycline class drug that is administered intravenously. It was developed in response to the growing rate of antibiotic resistant bacteria such as Staphylococcus aureus, Acinetobacter baumannii, and E. coli. As a tetracycline derivative antibiotic, its structural modifications has expanded its therapeutic activity to include Gram-positive and Gram-negative organisms, including those of multi-drug resistance.

Tobramycin is an aminoglycoside antibiotic derived from Streptomyces tenebrarius that is used to treat various types of bacterial infections, particularly Gram-negative infections. It is especially effective against species of Pseudomonas.

Cefepime is a fourth-generation cephalosporin antibiotic. Cefepime has an extended spectrum of activity against Gram-positive and Gram-negative bacteria, with greater activity against both types of organism than third-generation agents. A 2007 meta-analysis suggested when data of trials were combined, mortality was increased in people treated with cefepime compared with other β-lactam antibiotics. In response, the U.S. Food and Drug Administration (FDA) performed their own meta-analysis which found no mortality difference.

Cefadroxil is a broad-spectrum antibiotic of the cephalosporin type, effective in Gram-positive and Gram-negative bacterial infections. It is a bactericidal antibiotic.

Lincomycin is a lincosamide antibiotic that comes from the actinomycete Streptomyces lincolnensis. A related compound, clindamycin, is derived from lincomycin by using thionyl chloride to replace the 7-hydroxy group with a chlorine atom with inversion of chirality. It was released for medical use in September 1964.

Marbofloxacin is a carboxylic acid derivative third generation fluoroquinolone antibiotic. It is used in veterinary medicine under the brand names Marbocyl, Forcyl, Marbo vet and Zeniquin. A formulation of marbofloxacin combined with clotrimazole and dexamethasone is available under the name Aurizon.

Cefotiam is a parenteral third-generation cephalosporin antibiotic. It has broad-spectrum activity against Gram-positive and Gram-negative bacteria. As a beta-lactam, its bactericidal activity results from the inhibition of cell wall synthesis via affinity for penicillin-binding proteins.

Cefoxitin is a second-generation cephamycin antibiotic developed by Merck & Co., Inc. from Cephamycin C in the year following its discovery, 1972. It was synthesized in order to create an antibiotic with a broader spectrum. It is often grouped with the second-generation cephalosporins. Cefoxitin requires a prescription and as of 2010 is sold under the brand name Mefoxin by Bioniche Pharma, LLC. The generic version of cefoxitin is known as cefoxitin sodium.

Cefoperazone is a third-generation cephalosporin antibiotic, marketed by Pfizer under the name Cefobid. It is one of few cephalosporin antibiotics effective in treating Pseudomonas bacterial infections which are otherwise resistant to these antibiotics.

Cefditoren, also known as cefditoren pivoxil is an antibiotic used to treat infections caused by Gram-positive and Gram-negative bacteria that are resistant to other antibiotics. It is mainly used for treatment of community acquired pneumonia. It is taken by mouth and is in the cephalosporin family of antibiotics, which is part of the broader beta-lactam group of antibiotics.
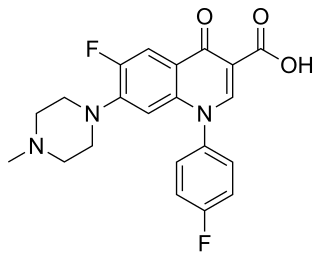
Difloxacin (INN), marketed under the trade name Dicural, is a second-generation, synthetic fluoroquinolone antibiotic used in veterinary medicine. It has broad-spectrum, concentration dependent, bactericidal activity; however, its efficacy is not as good as enrofloxacin or pradofloxacin.

Cefminox (INN) is a second-generation cephalosporin antibiotic.
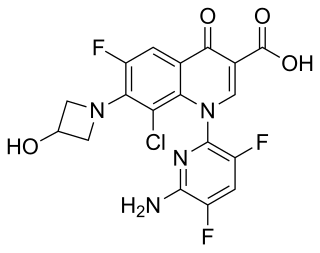
Delafloxacin sold under the brand name Baxdela among others, is a fluoroquinolone antibiotic used to treat acute bacterial skin and skin structure infections.
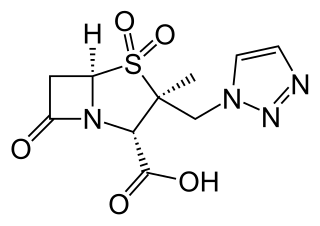
Ceftolozane/tazobactam, sold under the brand name Zerbaxa, is a fixed-dose combination antibiotic medication used for the treatment of complicated urinary tract infections and complicated intra-abdominal infections in adults. Ceftolozane is a cephalosporin antibiotic, developed for the treatment of infections with gram-negative bacteria that are resistant to conventional antibiotics. It was studied for urinary tract infections, intra-abdominal infections and ventilator-associated bacterial pneumonia.

Finafloxacin (Xtoro) is a fluoroquinolone antibiotic. In the United States, it is approved by the Food and Drug Administration to treat acute otitis externa caused by the bacteria Pseudomonas aeruginosa and Staphylococcus aureus.

Murepavadin also known as POL7080 is a Pseudomonas specific peptidomimetic antibiotic. It is a synthetic cyclic beta hairpin peptidomimetic based on the cationic antimicrobial peptide protegrin I (PG-1) and the first example of an outer membrane protein-targeting antibiotic class with a novel, nonlytic mechanism of action, highly active and selective against the protein transporter LptD of Pseudomonas aeruginosa. In preclinical studies the compound was highly active on a broad panel of clinical isolates including multi-drug resistant Pseudomonas bacteria with outstanding in vivo efficacy in sepsis, lung, and thigh infection models. Intravenous murepavadin is in development for the treatment of bacterial hospital-acquired pneumonia and bacterial ventilator-associated pneumonia due to Pseudomonas aeruginosa.




















