
Grubbs catalysts are a series of transition metal carbene complexes used as catalysts for olefin metathesis. They are named after Robert H. Grubbs, the chemist who supervised their synthesis. Several generations of the catalyst have been developed. Grubbs catalysts tolerate many functional groups in the alkene substrates, are air-tolerant, and are compatible with a wide range of solvents. For these reasons, Grubbs catalysts have become popular in synthetic organic chemistry. Grubbs, together with Richard R. Schrock and Yves Chauvin, won the Nobel Prize in Chemistry in recognition of their contributions to the development of olefin metathesis.

Organoborane or organoboron compounds are chemical compounds of boron and carbon that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds.
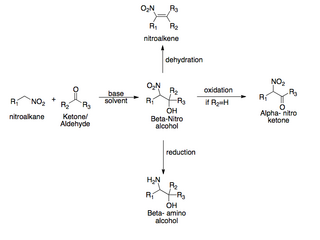
The Henry reaction is a classic carbon–carbon bond formation reaction in organic chemistry. Discovered in 1895 by the Belgian chemist Louis Henry (1834–1913), it is the combination of a nitroalkane and an aldehyde or ketone in the presence of a base to form β-nitro alcohols. This type of reaction is also referred to as a nitroaldol reaction. It is nearly analogous to the aldol reaction that had been discovered 23 years prior that couples two carbonyl compounds to form β-hydroxy carbonyl compounds known as "aldols". The Henry reaction is a useful technique in the area of organic chemistry due to the synthetic utility of its corresponding products, as they can be easily converted to other useful synthetic intermediates. These conversions include subsequent dehydration to yield nitroalkenes, oxidation of the secondary alcohol to yield α-nitro ketones, or reduction of the nitro group to yield β-amino alcohols.
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of the molecule.
In organic chemistry, the Arndt–Eistert reaction is the conversion of a carboxylic acid to its homologue. Named for the German chemists Fritz Arndt (1885–1969) and Bernd Eistert (1902–1978), the method entails treating an acid chlorides with diazomethane. It is a popular method of producing β-amino acids from α-amino acids.

A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the N-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for example diaminocarbenes with the general formula (R2N)2C:, where the four R moieties are typically alkyl and aryl groups. The groups can be linked to give heterocyclic carbenes, such as those derived from imidazole, imidazoline, thiazole or triazole.
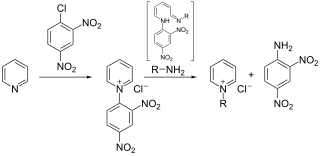
The Zincke reaction is an organic reaction, named after Theodor Zincke, in which a pyridine is transformed into a pyridinium salt by reaction with 2,4-dinitro-chlorobenzene and a primary amine.

In organic chemistry, organocatalysis is a form of catalysis in which the rate of a chemical reaction is increased by an organic catalyst. This "organocatalyst" consists of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds. Because of their similarity in composition and description, they are often mistaken as a misnomer for enzymes due to their comparable effects on reaction rates and forms of catalysis involved.

Ethylene dione or ethylenedione, also called dicarbon dioxide, Carbon peroxide, ethenedione, or ethene-1,2-dione, is a chemical compound with the formula C2O2 or O=C=C=O. It is an oxide of carbon, and can be described as the carbon-carbon covalent dimer of carbon monoxide. It can also be thought of as the dehydrated form of glyoxylic acid, or a ketone of ethenone H2C=C=O.

Anthony Joseph Arduengo III is Professor of the Practice at the Georgia Institute of Technology, Saxon Professor Emeritus of Chemistry at the University of Alabama, adjunct professor at the Institute for Inorganic Chemistry of Braunschweig University of Technology in Germany, and co-founder of the StanCE coalition for sustainable chemistry based on woody biomass. He is notable for his work on chemical compounds with unusual valency, especially in the field of stable carbene research.

Hans-Werner Wanzlick (1917-1988) was a German chemist. A Professor of chemistry at the Berlin Technical University he is notable for work on persistent carbenes and for proposing the Wanzlick equilibrium between saturated imidazolin-2-ylidenes and their dimers — which he called "das doppelte Lottchen", after a 1949 novel by Erich Kästner about a pair of mischievous twins.

Dihydroimidazol-2-ylidene is a hypothetical organic compound with formula C3H6N2. It would be a heterocyclic compound, formally derived from imidazolidine with two hydrogen atoms removed from carbon number 2, leaving two vacant chemical bonds — which makes it a carbene.
Michael K. Denk is a Professor of chemistry at the University of Guelph, Ontario.
Münchnone (synonyms: 1,3-oxazolium-5-oxide; 1,3-oxazolium-5-olate; anhydro-5-hydroxy-1,3-oxazolium hydroxide; 5-hydroxy-1,3-oxazolium hydroxide, inner salt; oxido-oxazolium) is a mesoionic heterocyclic aromatic chemical compound, with the molecular formula C3H3NO2. The name refers to the city of Munich, Germany (German: München), where the compound and its derivatives were first discovered and studied.
Carbene dimerization is a type of organic reaction in which two carbene or carbenoid precursors react in a formal dimerization to an alkene. This reaction is often considered an unwanted side-reaction but it is also investigated as a synthetic tool. In this reaction type either the two carbenic intermediates react or a carbenic intermediate reacts with a carbene precursor. An early pioneer was Christoph Grundmann reporting on a carbene dimerisation in 1938. In the domain of persistent carbenes the Wanzlick equilibrium describes an equilibrium between a carbene and its alkene.
David Markham Lemal is the Albert W. Smith Professor of Chemistry Emeritus and Research Professor of Chemistry at Dartmouth College. He received an A.B. degree (summa) from Amherst College in 1955 and a Ph.D. in Chemistry from Harvard University in 1959. At Harvard he worked with R. B. Woodward on deoxy sugars and a synthesis of the alkaloid yohimbine.

Plumbylenes (or plumbylidenes) are divalent organolead(II) analogues of carbenes, with the general chemical formula, R2Pb, where R denotes a substituent. Plumbylenes possess 6 electrons in their valence shell, and are considered open shell species.
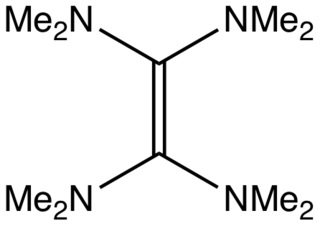
Tetrakis(dimethylamino)ethylene (TDAE) is an organic compound with the formula [C(NMe2)2]2 (where Me = CH3). A colorless liquid, this compound is classified as an enamine. Primary and secondary enamines tend to isomerize, but tertiary enamines are kinetically stable. The unusual feature of TDAE is that it is a tetra-enamine. The pi-donating tendency of the amine groups strongly modifies the properties of the molecule, which does exhibit properties of a typical alkene.
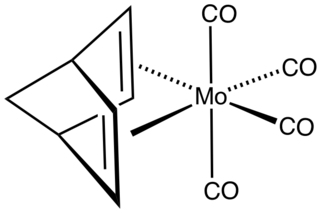
(Norbornadiene)molybdenum tetracarbonyl is the organomolybdenum compound with the formula (C7H9)Mo(CO)4. Structurally, the compound consists of the norbornadiene bonded to a Mo(CO)4 fragment. The compound is a yellow, volatile solid. It is prepared by thermal or photochemical substitution of molybdenum hexacarbonyl. The compound was originally examined as a potential antiknock agent.
Aluminium(I) nucleophiles are a group of inorganic and organometallic nucleophilic compounds containing at least one aluminium metal center in the +1 oxidation state with a lone pair of electrons strongly localized on the aluminium(I) center.















