
Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid–gas interfaces. It includes the fields of surface chemistry and surface physics. Some related practical applications are classed as surface engineering. The science encompasses concepts such as heterogeneous catalysis, semiconductor device fabrication, fuel cells, self-assembled monolayers, and adhesives. Surface science is closely related to interface and colloid science. Interfacial chemistry and physics are common subjects for both. The methods are different. In addition, interface and colloid science studies macroscopic phenomena that occur in heterogeneous systems due to peculiarities of interfaces.

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique based on the photoelectric effect that can identify the elements that exist within a material or are covering its surface, as well as their chemical state, and the overall electronic structure and density of the electronic states in the material. XPS is a powerful measurement technique because it not only shows what elements are present, but also what other elements they are bonded to. The technique can be used in line profiling of the elemental composition across the surface, or in depth profiling when paired with ion-beam etching. It is often applied to study chemical processes in the materials in their as-received state or after cleavage, scraping, exposure to heat, reactive gasses or solutions, ultraviolet light, or during ion implantation.

A synchrotron light source is a source of electromagnetic radiation (EM) usually produced by a storage ring, for scientific and technical purposes. First observed in synchrotrons, synchrotron light is now produced by storage rings and other specialized particle accelerators, typically accelerating electrons. Once the high-energy electron beam has been generated, it is directed into auxiliary components such as bending magnets and insertion devices in storage rings and free electron lasers. These supply the strong magnetic fields perpendicular to the beam that are needed to stimulate the high energy electrons to emit photons.
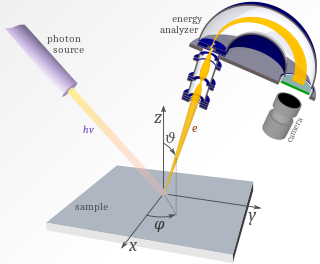
Photoemission spectroscopy (PES), also known as photoelectron spectroscopy, refers to energy measurement of electrons emitted from solids, gases or liquids by the photoelectric effect, in order to determine the binding energies of electrons in the substance. The term refers to various techniques, depending on whether the ionization energy is provided by X-ray, XUV or UV photons. Regardless of the incident photon beam, however, all photoelectron spectroscopy revolves around the general theme of surface analysis by measuring the ejected electrons.
Photoemission electron microscopy is a type of electron microscopy that utilizes local variations in electron emission to generate image contrast. The excitation is usually produced by ultraviolet light, synchrotron radiation or X-ray sources. PEEM measures the coefficient indirectly by collecting the emitted secondary electrons generated in the electron cascade that follows the creation of the primary core hole in the absorption process. PEEM is a surface sensitive technique because the emitted electrons originate from a shallow layer. In physics, this technique is referred to as PEEM, which goes together naturally with low-energy electron diffraction (LEED), and low-energy electron microscopy (LEEM). In biology, it is called photoelectron microscopy (PEM), which fits with photoelectron spectroscopy (PES), transmission electron microscopy (TEM), and scanning electron microscopy (SEM).

X-ray absorption fine structure (XAFS) is a specific structure observed in X-ray absorption spectroscopy (XAS). By analyzing the XAFS, information can be acquired on the local structure and on the unoccupied local electronic states.

Extended X-ray absorption fine structure (EXAFS), along with X-ray absorption near edge structure (XANES), is a subset of X-ray absorption spectroscopy (XAS). Like other absorption spectroscopies, XAS techniques follow Beer's law. The X-ray absorption coefficient of a material as a function of energy is obtained by directing X-rays of a narrow energy range at a sample, while recording the incident and transmitted x-ray intensity, as the incident x-ray energy is incremented.
X-ray absorption near edge structure (XANES), also known as near edge X-ray absorption fine structure (NEXAFS), is a type of absorption spectroscopy that indicates the features in the X-ray absorption spectra (XAS) of condensed matter due to the photoabsorption cross section for electronic transitions from an atomic core level to final states in the energy region of 50–100 eV above the selected atomic core level ionization energy, where the wavelength of the photoelectron is larger than the interatomic distance between the absorbing atom and its first neighbour atoms.
In X-ray absorption spectroscopy, the K-edge is a sudden increase in x-ray absorption occurring when the energy of the X-rays is just above the binding energy of the innermost electron shell of the atoms interacting with the photons. The term is based on X-ray notation, where the innermost electron shell is known as the K-shell. Physically, this sudden increase in attenuation is caused by the photoelectric absorption of the photons. For this interaction to occur, the photons must have more energy than the binding energy of the K-shell electrons (K-edge). A photon having an energy just above the binding energy of the electron is therefore more likely to be absorbed than a photon having an energy just below this binding energy or significantly above it.
X-ray Raman scattering (XRS) is non-resonant inelastic scattering of X-rays from core electrons. It is analogous to vibrational Raman scattering, which is a widely used tool in optical spectroscopy, with the difference being that the wavelengths of the exciting photons fall in the X-ray regime and the corresponding excitations are from deep core electrons.
Surface-extended X-ray absorption fine structure (SEXAFS) is the surface-sensitive equivalent of the EXAFS technique. This technique involves the illumination of the sample by high-intensity X-ray beams from a synchrotron and monitoring their photoabsorption by detecting in the intensity of Auger electrons as a function of the incident photon energy. Surface sensitivity is achieved by the interpretation of data depending on the intensity of the Auger electrons instead of looking at the relative absorption of the X-rays as in the parent method, EXAFS.
FEFF is a software program used in x-ray absorption spectroscopy. It contains self-consistent real space multiple-scattering code for simultaneous calculations of x-ray-absorption spectra and electronic structure. Output includes extended x-ray-absorption fine structure (EXAFS), full multiple scattering calculations of various x-ray absorption spectra (XAS) and projected local densities of states (LDOS). The spectra include x-ray absorption near edge structure (XANES), x-ray natural circular dichroism (XNCD), and non-resonant x-ray emission spectra. Calculations of the x-ray scattering amplitude and spin dependent calculations of x-ray magnetic circular dichroism (XMCD) and spin polarized x-ray absorption spectra are also possible, but less automated.

Metal L-edge spectroscopy is a spectroscopic technique used to study the electronic structures of transition metal atoms and complexes. This method measures X-ray absorption caused by the excitation of a metal 2p electron to unfilled d orbitals, which creates a characteristic absorption peak called the L-edge. Similar features can also be studied by Electron Energy Loss Spectroscopy. According to the selection rules, the transition is formally electric-dipole allowed, which not only makes it more intense than an electric-dipole forbidden metal K pre-edge transition, but also makes it more feature-rich as the lower required energy results in a higher-resolution experiment.

Resonant inelastic X-ray scattering (RIXS) is an advanced X-ray spectroscopy technique.
Operando spectroscopy is an analytical methodology wherein the spectroscopic characterization of materials undergoing reaction is coupled simultaneously with measurement of catalytic activity and selectivity. The primary concern of this methodology is to establish structure-reactivity/selectivity relationships of catalysts and thereby yield information about mechanisms. Other uses include those in engineering improvements to existing catalytic materials and processes and in developing new ones.
John J. Rehr is an American theoretical physicist, professor emeritus of physics at the University of Washington in Seattle. He has worked in the field of theoreticalX-ray and electron-spectroscopies.
Serena DeBeer is an American chemist. She is currently a W3-Professor and the director at the Max Planck Institute for Chemical Energy Conversion in Muelheim an der Ruhr, Germany, where she heads the Department of Inorganic Spectroscopy. Her expertise lies in the application and development of X-ray based spectroscopic methods as probes of electronic structure in biological and chemical catalysis.

SOLARIS is a synchrotron light source in the city of Kraków in Poland. It is the only one facility of its kind in Central-Eastern Europe. Built in 2015, under the auspices of the Jagiellonian University, it is located on the Campus of the 600th Anniversary of the Jagiellonian University Revival, in the southern part of the city. It is the central facility of the National Synchrotron Radiation Centre SOLARIS.

X-ray emission spectroscopy (XES) is a form of X-ray spectroscopy in which a core electron is excited by an incident x-ray photon and then this excited state decays by emitting an x-ray photon to fill the core hole. The energy of the emitted photon is the energy difference between the involved electronic levels. The analysis of the energy dependence of the emitted photons is the aim of the X-ray emission spectroscopy.









