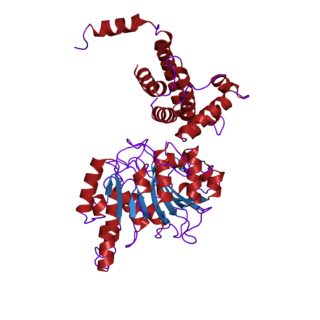
In biochemistry, the DNA methyltransferase family of enzymes catalyze the transfer of a methyl group to DNA. DNA methylation serves a wide variety of biological functions. All the known DNA methyltransferases use S-adenosyl methionine (SAM) as the methyl donor.

Ribosomal DNA (rDNA) is a DNA sequence that codes for ribosomal RNA. These sequences regulate transcription initiation and amplification, and contain both transcribed and non-transcribed spacer segments.

Ribosomal ribonucleic acid (rRNA) is a type of non-coding RNA which is the primary component of ribosomes, essential to all cells. rRNA is a ribozyme which carries out protein synthesis in ribosomes. Ribosomal RNA is transcribed from ribosomal DNA (rDNA) and then bound to ribosomal proteins to form small and large ribosome subunits. rRNA is the physical and mechanical factor of the ribosome that forces transfer RNA (tRNA) and messenger RNA (mRNA) to process and translate the latter into proteins. Ribosomal RNA is the predominant form of RNA found in most cells; it makes up about 80% of cellular RNA despite never being translated into proteins itself. Ribosomes are composed of approximately 60% rRNA and 40% ribosomal proteins by mass.

Methyltransferases are a large group of enzymes that all methylate their substrates but can be split into several subclasses based on their structural features. The most common class of methyltransferases is class I, all of which contain a Rossmann fold for binding S-Adenosyl methionine (SAM). Class II methyltransferases contain a SET domain, which are exemplified by SET domain histone methyltransferases, and class III methyltransferases, which are membrane associated. Methyltransferases can also be grouped as different types utilizing different substrates in methyl transfer reactions. These types include protein methyltransferases, DNA/RNA methyltransferases, natural product methyltransferases, and non-SAM dependent methyltransferases. SAM is the classical methyl donor for methyltransferases, however, examples of other methyl donors are seen in nature. The general mechanism for methyl transfer is a SN2-like nucleophilic attack where the methionine sulfur serves as the leaving group and the methyl group attached to it acts as the electrophile that transfers the methyl group to the enzyme substrate. SAM is converted to S-Adenosyl homocysteine (SAH) during this process. The breaking of the SAM-methyl bond and the formation of the substrate-methyl bond happen nearly simultaneously. These enzymatic reactions are found in many pathways and are implicated in genetic diseases, cancer, and metabolic diseases. Another type of methyl transfer is the radical S-Adenosyl methionine (SAM) which is the methylation of unactivated carbon atoms in primary metabolites, proteins, lipids, and RNA.

A ribosomal protein L21 leader is a ribosomal protein leader autoregulatory structure that regulates mRNAs containing a gene that encodes ribosomal protein L21. An RNA motif was predicted to function as an L21 leader in a bioinformatics study, and is found in B. subtilis and other low-GC Gram-positive bacteria within the phylum Bacillota. It is located in the 5′ untranslated regions of mRNAs encoding ribosomal protein L21, a protein of unknown function, and ribosomal protein L27 (rplU-ysxB-rpmA).

In enzymology, a protein-glutamate O-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a rRNA (adenine-N6-)-methyltransferase (EC 2.1.1.48) is an enzyme that catalyzes the chemical reaction

16S ribosomal RNA is the RNA component of the 30S subunit of a prokaryotic ribosome. It binds to the Shine-Dalgarno sequence and provides most of the SSU structure.

The 23S rRNA is a 2,904 nucleotide long component of the large subunit (50S) of the bacterial/archean ribosome and makes up the peptidyl transferase center (PTC). The 23S rRNA is divided into six secondary structural domains titled I-VI, with the corresponding 5S rRNA being considered domain VII. The ribosomal peptidyl transferase activity resides in domain V of this rRNA, which is also the most common binding site for antibiotics that inhibit translation, making it a target for ribosomal engineering. A well-known member of this antibiotic class, chloramphenicol, acts by inhibiting peptide bond formation, with recent 3D-structural studies showing two different binding sites depending on the species of ribosome. Numerous mutations in domains of the 23S rRNA with Peptidyl transferase activity have resulted in antibiotic resistance. 23S rRNA genes typically have higher sequence variations, including insertions and/or deletions, compared to other rRNAs.

The sucA RNA motif is a conserved RNA structure found in bacteria of the order Burkholderiales. RNAs within this motif are always found in the presumed 5' UTR of sucA genes. sucA encodes a subunit of an enzyme that participates in the citric acid cycle by synthesizing succinyl-CoA from 2-oxoglutarate. A part of the conserved structure overlaps predicted Shine-Dalgarno sequences of the downstream sucA genes. Because of the RNA motif's consistent gene association and a possible mechanism for sequestering the ribosome binding site, it was proposed that the sucA RNA motif corresponds to a cis-regulatory element. Its relatively complex secondary structure could indicate that it is a riboswitch. However, the function of this RNA motif remains unknown.
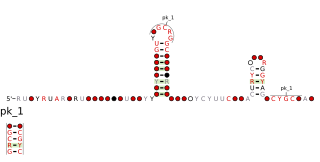
The Cyano-S1 RNA motif is a conserved RNA structure present in some species of Cyanobacteria. Cyano-S1 RNAs are consistently found upstream of genes encoding ribosomal protein S1, a subunit of the ribosome. Therefore, they are presumed to be ribosomal protein leaders, i.e., cis-regulatory elements to which the ribosomal protein S1 binds, thereby controlling its expression levels.

The glutamine riboswitch is a conserved RNA structure that was predicted by bioinformatics. It is present in a variety of lineages of cyanobacteria, as well as some phages that infect cyanobacteria. It is also found in DNA extracted from uncultivated bacteria living in the ocean that are presumably species of cyanobacteria.

The L17 downstream element RNA motif is a conserved RNA structure identified in bacteria by bioinformatics. All known L17 downstream elements were detected immediately downstream of genes encoding the L17 subunit of the ribosome, and therefore might be in the 3' untranslated regions of these genes. The element is found in a variety of lactic acid bacteria and in the genus Listeria.

The rmf RNA motif is a conserved RNA structure that was originally detected using bioinformatics. rmf RNAs are consistently foundwithin species classified into the genus Pseudomonas, and is located potentially in the 5′ untranslated regions of rmf genes. These genes encodes the ribosome modulation factor protein, which affects the translation of genes by modifying ribosome structure in response to stress such as starvation. This ribosome modulation is a part of the stringent response in bacteria. The likely biological role of rmf RNAs is ambiguous. Since the RNA could be in the 5′ UTRs of protein-coding genes, it was hypothesized that it functions as a cis-regulatory element. This hypothesis is bolstered by the observation that ribosome modulation factor binds ribosomal RNA, and many cis-regulatory RNAs called ribosomal protein leaders participate in a feedback regulation mechanism by binding to proteins that normally bind to ribosomal RNA. However, since rmf RNAs are not very close to the rmf genes, they might function as non-coding RNAs.
23S rRNA (adenine1618-N6)-methyltransferase (EC 2.1.1.181, rRNA large subunit methyltransferase F, YbiN protein, rlmF (gene), m6A1618 methyltransferase) is an enzyme with systematic name S-adenosyl-L-methionine:23S rRNA (adenine1618-N6)-methyltransferase. This enzyme catalyses the following chemical reaction
23S rRNA (guanine745-N1)-methyltransferase (EC 2.1.1.187, Rlma(I), Rlma1, 23S rRNA m1G745 methyltransferase, YebH, RlmAI methyltransferase, ribosomal RNA(m1G)-methylase, rRNA(m1G)methylase, RrmA, 23S rRNA:m1G745 methyltransferase) is an enzyme with systematic name S-adenosyl-L-methionine:23S rRNA (guanine745-N1)-methyltransferase. This enzyme catalyses the following chemical reaction
23S rRNA (adenine2503-C2,C8)-dimethyltransferase (EC 2.1.1.194, Cfr, Cfr methyltransferase, Cfr rRNA methyltransferase) is an enzyme with systematic name S-adenosyl-L-methionine:23S rRNA (adenine2503-C2,C8)-dimethyltransferase. This enzyme catalyses the following chemical reaction
23S rRNA (adenine2503-C8)-methyltransferase (EC 2.1.1.224, Cfr (gene)) is an enzyme with systematic name S-adenosyl-L-methionine:23S rRNA (adenine2503-C8)-methyltransferase. This enzyme catalyses the following chemical reaction
23S rRNA (adenosine1067-2'-O)-methyltransferase is an enzyme with systematic name S-adenosyl-L-methionine:23S rRNA (adenosine1067-2'-O)-methyltransferase. This enzyme catalyses the following chemical reaction













