Related Research Articles
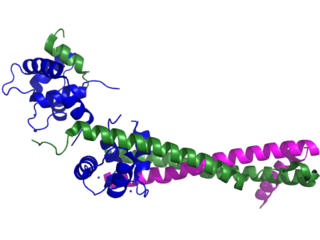
Troponin, or the troponin complex, is a complex of three regulatory proteins that are integral to muscle contraction in skeletal muscle and cardiac muscle, but not smooth muscle. Measurements of cardiac-specific troponins I and T are extensively used as diagnostic and prognostic indicators in the management of myocardial infarction and acute coronary syndrome. Blood troponin levels may be used as a diagnostic marker for stroke or other myocardial injury that is ongoing, although the sensitivity of this measurement is low.
Hypertrophic cardiomyopathy is a condition in which the heart becomes thickened without an obvious cause. The parts of the heart most commonly affected are the interventricular septum and the ventricles. This results in the heart being less able to pump blood effectively and also may cause electrical conduction problems.
Troponin C is a protein which is part of the troponin complex. It contains four calcium-binding EF hands, although different isoforms may have fewer than four functional calcium-binding subdomains. It is a component of thin filaments, along with actin and tropomyosin. It contains an N lobe and a C lobe. The C lobe serves a structural purpose and binds to the N domain of troponin I (TnI). The C lobe can bind either Ca2+ or Mg2+. The N lobe, which binds only Ca2+, is the regulatory lobe and binds to the C domain of troponin I after calcium binding.

Troponin I, cardiac muscle is a protein that in humans is encoded by the TNNI3 gene. It is a tissue-specific subtype of troponin I, which in turn is a part of the troponin complex.

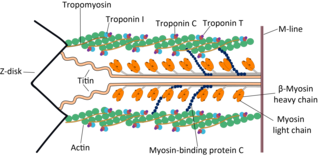
Cardiac muscle troponin T (cTnT) is a protein that in humans is encoded by the TNNT2 gene. Cardiac TnT is the tropomyosin-binding subunit of the troponin complex, which is located on the thin filament of striated muscles and regulates muscle contraction in response to alterations in intracellular calcium ion concentration.

Ryanodine receptor 2 (RYR2) is one of a class of ryanodine receptors and a protein found primarily in cardiac muscle. In humans, it is encoded by the RYR2 gene. In the process of cardiac calcium-induced calcium release, RYR2 is the major mediator for sarcoplasmic release of stored calcium ions.

Tropomyosin alpha-1 chain is a protein that in humans is encoded by the TPM1 gene. This gene is a member of the tropomyosin (Tm) family of highly conserved, widely distributed actin-binding proteins involved in the contractile system of striated and smooth muscles and the cytoskeleton of non-muscle cells.

The myosin-binding protein C, cardiac-type is a protein that in humans is encoded by the MYBPC3 gene. This isoform is expressed exclusively in heart muscle during human and mouse development, and is distinct from those expressed in slow skeletal muscle (MYBPC1) and fast skeletal muscle (MYBPC2).

Troponin C, also known as TN-C or TnC, is a protein that resides in the troponin complex on actin thin filaments of striated muscle and is responsible for binding calcium to activate muscle contraction. Troponin C is encoded by the TNNC1 gene in humans for both cardiac and slow skeletal muscle.

β-Tropomyosin, also known as tropomyosin beta chain is a protein that in humans is encoded by the TPM2 gene. β-tropomyosin is striated muscle-specific coiled coil dimer that functions to stabilize actin filaments and regulate muscle contraction.

Telethonin, also known as Tcap, is a protein that in humans is encoded by the TCAP gene. Telethonin is expressed in cardiac and skeletal muscle at Z-discs and functions to regulate sarcomere assembly, T-tubule function and apoptosis. Telethonin has been implicated in several diseases, including limb-girdle muscular dystrophy, hypertrophic cardiomyopathy, dilated cardiomyopathy and idiopathic cardiomyopathy.

Troponin I, slow skeletal muscle is a protein that in humans is encoded by the TNNI1 gene. It is a tissue-specific subtype of troponin I, which in turn is a part of the troponin complex.

Troponin I, fast skeletal muscle is a protein that in humans is encoded by the TNNI2 gene.

Myosin regulatory light chain 2, ventricular/cardiac muscle isoform (MLC-2) also known as the regulatory light chain of myosin (RLC) is a protein that in humans is encoded by the MYL2 gene. This cardiac ventricular RLC isoform is distinct from that expressed in skeletal muscle (MYLPF), smooth muscle (MYL12B) and cardiac atrial muscle (MYL7).

Slow skeletal muscle troponin T (sTnT) is a protein that in humans is encoded by the TNNT1 gene.

Myosin heavy chain, α isoform (MHC-α) is a protein that in humans is encoded by the MYH6 gene. This isoform is distinct from the ventricular/slow myosin heavy chain isoform, MYH7, referred to as MHC-β. MHC-α isoform is expressed predominantly in human cardiac atria, exhibiting only minor expression in human cardiac ventricles. It is the major protein comprising the cardiac muscle thick filament, and functions in cardiac muscle contraction. Mutations in MYH6 have been associated with late-onset hypertrophic cardiomyopathy, atrial septal defects and sick sinus syndrome.

Myosin essential light chain (ELC), ventricular/cardiac isoform is a protein that in humans is encoded by the MYL3 gene. This cardiac ventricular/slow skeletal ELC isoform is distinct from that expressed in fast skeletal muscle (MYL1) and cardiac atrial muscle (MYL4). Ventricular ELC is part of the myosin molecule and is important in modulating cardiac muscle contraction.
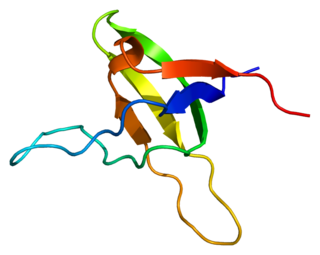
Obscurin is a protein that in humans is encoded by the OBSCN gene. Obscurin belongs to the family of giant sarcomeric signaling proteins that includes titin and nebulin. Obscurin is expressed in cardiac and skeletal muscle, and plays a role in the organization of myofibrils during sarcomere assembly. A mutation in the OBSCN gene has been associated with hypertrophic cardiomyopathy and altered obscurin protein properties have been associated with other muscle diseases.
Bernhard Brenner is a scientist who, through his experiments, elucidated the repeated cycles of stretch and release of muscle fibers under isometric conditions. Because of this, these cycles were named as "Brenner Cycles".
D145E is a point mutation on troponin C that leads to hypertrophic cardiomyopathy disease. This mutation is caused by the change of nucleotide C to A at nucleotide 435, switching the amino acid aspartic acid to glutamic acid, which is located at the C-terminal tail. Patients with this mutation have different structure on the thin filament and alter the binding of Ca2+ at the troponin C site IV. Further, D145E causes increase in development of force and activation of ATPase in the presence of Ca2+.
References
- ↑ Ohtsuki, Iwao; Morimoto, Sachio (April 2008). "Troponin: Regulatory function and disorders". Biochemical and Biophysical Research Communications. 369 (1): 62–73. doi:10.1016/j.bbrc.2007.11.187. PMID 18154728.
- ↑ Solaro, R. John; Rosevear, Paul; Kobayashi, Tomoyoshi (April 2008). "The unique functions of cardiac troponin I in the control of cardiac muscle contraction and relaxation". Biochemical and Biophysical Research Communications. 369 (1): 82–87. doi:10.1016/j.bbrc.2007.12.114. PMC 2365727 . PMID 18162178.
- 1 2 Kalyva, Athanasia; Parthenakis, Fragiskos I.; Marketou, Maria E.; Kontaraki, Joanna E.; Vardas, Panos E. (April 2014). "Biochemical characterisation of Troponin C mutations causing hypertrophic and dilated cardiomyopathies". Journal of Muscle Research and Cell Motility. 35 (2): 161–178. doi:10.1007/s10974-014-9382-0. ISSN 0142-4319. PMID 24744096. S2CID 1726747.
- 1 2 Pinto, Jose Renato; Parvatiyar, Michelle S.; Jones, Michelle A.; Liang, Jingsheng; Ackerman, Michael J.; Potter, James D. (2009-07-10). "A Functional and Structural Study of Troponin C Mutations Related to Hypertrophic Cardiomyopathy". Journal of Biological Chemistry. 284 (28): 19090–19100. doi: 10.1074/jbc.M109.007021 . ISSN 0021-9258. PMC 2707221 . PMID 19439414.