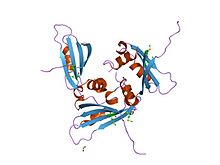
Streptomyces is the largest genus of Actinomycetota, and the type genus of the family Streptomycetaceae. Over 700 species of Streptomyces bacteria have been described. As with the other Actinomycetota, streptomycetes are gram-positive, and have very large genomes with high GC content. Found predominantly in soil and decaying vegetation, most streptomycetes produce spores, and are noted for their distinct "earthy" odor that results from production of a volatile metabolite, geosmin. Different strains of the same species may colonize very diverse environments.

Viomycin is a member of the tuberactinomycin family, a group of nonribosomal peptide antibiotics exhibiting anti-tuberculosis activity. The tuberactinomycin family is an essential component in the drug cocktail currently used to fight infections of Mycobacterium tuberculosis. Viomycin was the first member of the tuberactinomycins to be isolated and identified, and was used to treat TB until it was replaced by the less toxic, but structurally related compound, capreomycin. The tuberactinomycins target bacterial ribosomes, binding RNA and disrupting bacterial protein synthesis and certain forms of RNA splicing. Viomycin is produced by the actinomycete Streptomyces puniceus.

Novobiocin, also known as albamycin, is an aminocoumarin antibiotic that is produced by the actinomycete Streptomyces niveus, which has recently been identified as a subjective synonym for S. spheroides a member of the class Actinomycetia. Other aminocoumarin antibiotics include clorobiocin and coumermycin A1. Novobiocin was first reported in the mid-1950s.

Perosamine is a mannose-derived 4-aminodeoxysugar produced by some bacteria.

Doxorubicin (DXR) is a 14-hydroxylated version of daunorubicin, the immediate precursor of DXR in its biosynthetic pathway. Daunorubicin is more abundantly found as a natural product because it is produced by a number of different wild type strains of Streptomyces. In contrast, only one known non-wild type species, Streptomyces peucetius subspecies caesius ATCC 27952, was initially found to be capable of producing the more widely used doxorubicin. This strain was created by Arcamone et al. in 1969 by mutating a strain producing daunorubicin, but not DXR, at least in detectable quantities. Subsequently, Hutchinson's group showed that under special environmental conditions, or by the introduction of genetic modifications, other strains of streptomyces can produce doxorubicin. His group has also cloned many of the genes required for DXR production, although not all of them have been fully characterized. In 1996, Strohl's group discovered, isolated and characterized dox A, the gene encoding the enzyme that converts daunorubicin into DXR. By 1999, they produced recombinant Dox A, a Cytochrome P450 oxidase, and found that it catalyzes multiple steps in DXR biosynthesis, including steps leading to daunorubicin. This was significant because it became clear that all daunorubicin producing strains have the necessary genes to produce DXR, the much more therapeutically important of the two. Hutchinson's group went on to develop methods to improve the yield of DXR, from the fermentation process used in its commercial production, not only by introducing Dox A encoding plasmids, but also by introducing mutations to deactivate enzymes that shunt DXR precursors to less useful products, for example baumycin-like glycosides. Some triple mutants, that also over-expressed Dox A, were able to double the yield of DXR. This is of more than academic interest because at that time DXR cost about $1.37 million per kg and current production in 1999 was 225 kg per annum. More efficient production techniques have brought the price down to $1.1 million per kg for the non-liposomal formulation. Although DXR can be produced semi-synthetically from daunorubicin, the process involves electrophilic bromination and multiple steps and the yield is poor. Since daunorubicin is produced by fermentation, it would be ideal if the bacteria could complete DXR synthesis more effectively.

Aminocoumarin is a class of antibiotics that act by an inhibition of the DNA gyrase enzyme involved in the cell division in bacteria. They are derived from Streptomyces species, whose best-known representative – Streptomyces coelicolor – was completely sequenced in 2002. The aminocoumarin antibiotics include:
In enzymology, a [acyl-carrier-protein] S-malonyltransferase is an enzyme that catalyzes the chemical reaction

Thiostrepton is a natural cyclic oligopeptide antibiotic of the thiopeptide class, derived from several strains of streptomycetes, such as Streptomyces azureus and Streptomyces laurentii. Thiostrepton is a natural product of the ribosomally synthesized and post-translationally modified peptide (RiPP) class.

6C RNA is a class of non-coding RNA present in actinomycetes. 6C RNA was originally discovered as a conserved RNA structure having two stem-loops each containing six or more cytosine (C) residues. Later work revealed that 6C RNAs in Streptomyces coelicolor and Streptomyces avermitilis have predicted rho-independent transcription terminators, and microarray and reverse-transcriptase PCR experiments indicate that the S. coelicolor version is transcribed as RNA. Transcription of the S. coelicolor RNA increases during sporulation, and three transcripts were detected that overlap the 6C motif, but have different apparent start and stop sites.

In molecular biology, the polyketide synthesis cyclase family of proteins includes a number of cyclases involved in polyketide synthesis in a number of actinobacterial species.
Epi-isozizaene 5-monooxygenase (EC 1.14.13.106, CYP170A1) is an enzyme with systematic name (+)-epi-isozizaene,NADPH:oxygen oxidoreductase (5-hydroxylating). This enzyme catalyses the following chemical reaction

Geosmin synthase or germacradienol-geosmin synthase designates a class of bifunctional enzymes that catalyze the conversion of farnesyl diphosphate (FPP) to geosmin, a volatile organic compound known for its earthy smell. The N-terminal half of the protein catalyzes the conversion of farnesyl diphosphate to germacradienol and germacrene D, followed by the C-terminal-mediated conversion of germacradienol to geosmin. The conversion of FPP to geosmin was previously thought to involve multiple enzymes in a biosynthetic pathway.
Germacradienol synthase (EC 4.2.3.22, germacradienol/germacrene-D synthase, 2-trans,6-trans-farnesyl-diphosphate diphosphate-lyase [(1E,4S,5E,7R)-germacra-1(10),5-dien-11-ol-forming]) is an enzyme with systematic name (2E,6E)-farnesyl-diphosphate diphosphate-lyase ((1E,4S,5E,7R)-germacra-1(10),5-dien-11-ol-forming). This enzyme catalyses the following chemical reaction
epi-Isozizaene synthase is an enzyme with systematic name (2E,6E)-farnesyl-diphosphate diphosphate-lyase ( -epi-isozizaene-forming). This enzyme catalyses the following chemical reaction
Streptomyces isolates have yielded the majority of human, animal, and agricultural antibiotics, as well as a number of fundamental chemotherapy medicines. Streptomyces is the largest antibiotic-producing genus of Actinomycetota, producing chemotherapy, antibacterial, antifungal, antiparasitic drugs, and immunosuppressants. Streptomyces isolates are typically initiated with the aerial hyphal formation from the mycelium.
Streptomyces albidoflavus is a bacterium species from the genus of Streptomyces which has been isolated from soil from Poland. Streptomyces albidoflavus produces dibutyl phthalate and streptothricins.
Streptomyces nogalater is a bacterium species from the genus of Streptomyces. Streptomyces nogalater produces nogalamycin.
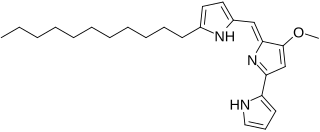
Undecylprodigiosin is an alkaloid produced by some Actinomycetes bacteria. It is a member of the prodiginines group of natural products and has been investigated for potential antimalarial activity.
Cytochrome P450, family 105, also known as CYP105, is a cytochrome P450 monooxygenase family in bacteria, predominantly found in the phylum Actinomycetota and the order Actinomycetales. The first three genes and subfamilies identified in this family is the herbicide-inducible P-450SU1 and P-450SU2 from Streptomyces griseolus and choP from Streptomyces sp's cholesterol oxidase promoter region.
Cytochrome P450, family 107, also known as CYP107, is a cytochrome P450 monooxygenase family in bacteria, found to be conserved and highly populated in Streptomyces and Bacillus species. The first gene identified in this family is Cytochrome P450 eryF (CYP107A1) from Saccharopolyspora erythraea. Many enzymes of this family are involved in the synthesis of macrolide antibiotics. The members of this family are widely distributed in Alphaproteobacteria, cyanobacterial, Mycobacterium, Bacillota, and Streptomyces species, which may be due to horizontal gene transfer driven by selection pressure.