
Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on scratch hardness comparison. Large calcite crystals are used in optical equipment, and limestone composed mostly of calcite has numerous uses.

Natron is a naturally occurring mixture of sodium carbonate decahydrate (Na2CO3·10H2O, a kind of soda ash) and around 17% sodium bicarbonate (also called baking soda, NaHCO3) along with small quantities of sodium chloride and sodium sulfate. Natron is white to colourless when pure, varying to gray or yellow with impurities. Natron deposits are sometimes found in saline lake beds which arose in arid environments. Throughout history natron has had many practical applications that continue today in the wide range of modern uses of its constituent mineral components.

Malachite is a copper carbonate hydroxide mineral, with the formula Cu2CO3(OH)2. This opaque, green-banded mineral crystallizes in the monoclinic crystal system, and most often forms botryoidal, fibrous, or stalagmitic masses, in fractures and deep, underground spaces, where the water table and hydrothermal fluids provide the means for chemical precipitation. Individual crystals are rare, but occur as slender to acicular prisms. Pseudomorphs after more tabular or blocky azurite crystals also occur.

Wulfenite is a lead molybdate mineral with the formula PbMoO4. It can be most often found as thin tabular crystals with a bright orange-red to yellow-orange color, sometimes brown, although the color can be highly variable. In its yellow form it is sometimes called "yellow lead ore".

Dawsonite is a mineral composed of sodium aluminium carbonate hydroxide, chemical formula NaAlCO3(OH)2. It crystallizes in the orthorhombic crystal system. It is not mined for ore. It was discovered in 1874 during the construction of the Redpath Museum in a feldspathic dike on the campus of McGill University on the Island of Montreal, Canada. It is named after geologist Sir John William Dawson (1820–1899).
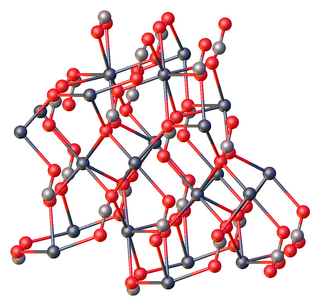
Lead(II) carbonate is the chemical compound with the chemical formula PbCO3. It is a white solid with several practical uses, despite its toxicity. It occurs naturally as the mineral cerussite.

Andersonite, Na2Ca(UO2)(CO3)3·6H2O, or hydrated sodium calcium uranyl carbonate is a rare uranium carbonate mineral that was first described in 1948. Named after Charles Alfred Anderson (1902–1990) of the United States Geological Survey, who first described the mineral species, it is found in sandstone-hosted uranium deposits. It has a high vitreous to pearly luster and is fluorescent. Andersonite specimens will usually glow a bright lemon yellow (or green with blue hints depending on the deposit) in ultraviolet light. It is commonly found as translucent small rhombohedral crystals that have angles close to 90 degrees although its crystal system is nominally trigonal. Its Mohs hardness is 2.5, with an average specific gravity of 2.8.

Duftite is a relatively common arsenate mineral with the formula CuPb(AsO4)(OH), related to conichalcite. It is green and often forms botryoidal aggregates. It is a member of the adelite-descloizite Group, Conichalcite-Duftite Series. Duftite and conichalcite specimens from Tsumeb are commonly zoned in color and composition. Microprobe analyses and X-ray powder-diffraction studies indicate extensive substitution of Zn for Cu, and Ca for Pb in the duftite structure. This indicates a solid solution among conichalcite, CaCu(AsO4 )(OH), austinite, CaZn(AsO4)(OH) and duftite PbCu(AsO4)(OH), all of them belonging to the adelite group of arsenates. It was named after Mining Councilor G Duft, Director of the Otavi Mine and Railroad Company, Tsumeb, Namibia. The type locality is the Tsumeb Mine, Tsumeb, Otjikoto Region, Namibia.

Matlockite is a rare lead halide mineral, named after the town of Matlock in Derbyshire, England, where it was first discovered in a nearby mine. Matlockite gives its name to the matlockite group which consists of rare minerals of a similar structure.

Susannite is a lead sulfate carbonate hydroxide mineral. It has the formula Pb4SO4(CO3)2(OH)2. Susannite is the higher temperature phase of the two and forms above 80 °C when fluids oxidize the lead ore deposits. It is trimorphous with leadhillite and macphersonite.
Macphersonite, Pb4(SO4)(CO3)2 (OH)2, is a carbonate mineral that is trimorphous with leadhillite and susannite. Macphersonite is generally white, colorless, or a pale amber in color and has a white streak. It crystallizes in the orthorhombic system with a space group of Pcab. It is fairly soft mineral that has a high specific gravity.
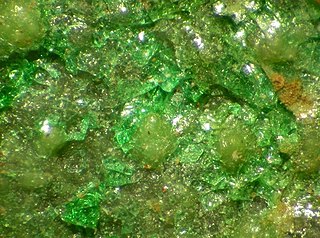
Widgiemoolthalite is a rare hydrated nickel(II) carbonate mineral with the chemical formula (Ni,Mg)5(CO3)4(OH)2·5H2O. Usually bluish-green in color, it is a brittle mineral formed during the weathering of nickel sulfide. Present on gaspéite surfaces, widgiemoolthalite has a Mohs scale hardness of 3.5 and an unknown though likely disordered crystal structure. Widgiemoolthalite was first discovered in 1992 in Widgiemooltha, Western Australia, which is to date its only known source. It was named the following year by the three researchers who first reported its existence, Ernest H. Nickel, Bruce W. Robinson, and William G. Mumme.
The Carbon Mineral Challenge is a citizen science project dedicated to accelerating the discovery of carbon-bearing minerals. The program launched in 2015 December with sponsorship from the Deep Carbon Observatory. The project ended in 2019 September, with 31 new carbon-bearing minerals found from 27 locations.
Braunerite is a hydrous uranyl carbonate mineral discovered by Jakub Plášil of the Institute of Physics at the Academy of Sciences of the Czech Republic and colleagues in the Svornost mine in the Jáchymov ore district, Western Bohemia, Czech Republic.
Marklite is a hydrated copper carbonate mineral named after Gregor Markl, a German mineralogist at the University of Tübingen. Markl found the type specimen of marklite in the dumps of the Friedrich-Christian mine in the Black Forest Mountains in southwestern Germany. Markl specializes in crustal petrology and geochemistry and has studied the hydrothermal ore deposits of the Black Forest area. Jakub Plášil of the Institute of Physics at the Academy of Sciences of the Czech Republic and colleagues identified its structure.

Joan Abella i Creus is a Catalan gemmologist and mineralogist who discovered abellaite, a mineral that receives this name in his honor

Mineral evolution is a recent hypothesis that provides historical context to mineralogy. It postulates that mineralogy on planets and moons becomes increasingly complex as a result of changes in the physical, chemical and biological environment. In the Solar System, the number of mineral species has grown from about a dozen to over 5400 as a result of three processes: separation and concentration of elements; greater ranges of temperature and pressure coupled with the action of volatiles; and new chemical pathways provided by living organisms.

Lamprophyllite is a rare, but widespread mineral Ti-silicate mineral usually found in intrusive agpasitic igneous rocks. Yellow, reddish brown, Vitreous, Pearly.

A carbonate fluoride, fluoride carbonate, fluorocarbonate or fluocarbonate is a double salt containing both carbonate and fluoride. The salts are usually insoluble in water, and can have more than one kind of metal cation to make more complex compounds. Rare-earth fluorocarbonates are particularly important as ore minerals for the light rare-earth elements lanthanum, cerium and neodymium. Bastnäsite is the most important source of these elements. Other artificial compounds are under investigation as non-linear optical materials and for transparency in the ultraviolet, with effects over a dozen times greater than Potassium dideuterium phosphate.
The carbonate chlorides are double salts containing both carbonate and chloride anions. Quite a few minerals are known. Several artificial compounds have been made. Some complexes have both carbonate and chloride ligands. They are part of the family of halocarbonates. In turn these halocarbonates are a part of mixed anion materials.















