Related Research Articles

A spermatozoon is a motile sperm cell, or moving form of the haploid cell that is the male gamete. A spermatozoon joins an ovum to form a zygote.

The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components: microfilaments, intermediate filaments, and microtubules, and these are all capable of rapid growth or disassembly depending on the cell's requirements.
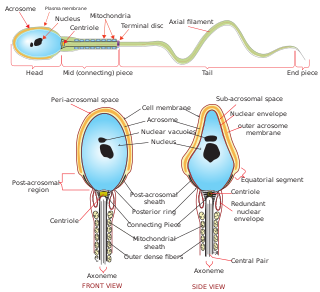
The acrosome is an organelle that develops over the anterior (front) half of the head in the spermatozoa of humans, and many other animals. It is a cap-like structure derived from the Golgi apparatus. In placental mammals, the acrosome contains degradative enzymes. These enzymes break down the outer membrane of the ovum, called the zona pellucida, allowing the haploid nucleus in the sperm cell to join with the haploid nucleus in the ovum. This shedding of the acrosome, or acrosome reaction, can be stimulated in vitro by substances a sperm cell may encounter naturally such as progesterone or follicular fluid, as well as the more commonly used calcium ionophore A23187. This can be done to serve as a positive control when assessing the acrosome reaction of a sperm sample by flow cytometry or fluorescence microscopy. This is usually done after staining with a fluoresceinated lectin such as FITC-PNA, FITC-PSA, FITC-ConA, or fluoresceinated antibody such as FITC-CD46.

Microfilaments, also called actin filaments, are protein filaments in the cytoplasm of eukaryotic cells that form part of the cytoskeleton. They are primarily composed of polymers of actin, but are modified by and interact with numerous other proteins in the cell. Microfilaments are usually about 7 nm in diameter and made up of two strands of actin. Microfilament functions include cytokinesis, amoeboid movement, cell motility, changes in cell shape, endocytosis and exocytosis, cell contractility, and mechanical stability. Microfilaments are flexible and relatively strong, resisting buckling by multi-piconewton compressive forces and filament fracture by nanonewton tensile forces. In inducing cell motility, one end of the actin filament elongates while the other end contracts, presumably by myosin II molecular motors. Additionally, they function as part of actomyosin-driven contractile molecular motors, wherein the thin filaments serve as tensile platforms for myosin's ATP-dependent pulling action in muscle contraction and pseudopod advancement. Microfilaments have a tough, flexible framework which helps the cell in movement.

Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of over 100 μM; its mass is roughly 42 kDa, with a diameter of 4 to 7 nm.

During fertilization, a sperm must first fuse with the plasma membrane and then penetrate the female egg cell to fertilize it. Fusing to the egg cell usually causes little problem, whereas penetrating through the egg's hard shell or extracellular matrix can be more difficult. Therefore, sperm cells go through a process known as the acrosome reaction, which is the reaction that occurs in the acrosome of the sperm as it approaches the egg.

Intermediate filaments (IFs) are cytoskeletal structural components found in the cells of vertebrates, and many invertebrates. Homologues of the IF protein have been noted in an invertebrate, the cephalochordate Branchiostoma.

Spermatogenesis is the process by which haploid spermatozoa develop from germ cells in the seminiferous tubules of the testicle. This process starts with the mitotic division of the stem cells located close to the basement membrane of the tubules. These cells are called spermatogonial stem cells. The mitotic division of these produces two types of cells. Type A cells replenish the stem cells, and type B cells differentiate into primary spermatocytes. The primary spermatocyte divides meiotically into two secondary spermatocytes; each secondary spermatocyte divides into two equal haploid spermatids by Meiosis II. The spermatids are transformed into spermatozoa (sperm) by the process of spermiogenesis. These develop into mature spermatozoa, also known as sperm cells. Thus, the primary spermatocyte gives rise to two cells, the secondary spermatocytes, and the two secondary spermatocytes by their subdivision produce four spermatozoa and four haploid cells.

The spermatid is the haploid male gametid that results from division of secondary spermatocytes. As a result of meiosis, each spermatid contains only half of the genetic material present in the original primary spermatocyte.
Capacitation is the penultimate step in the maturation of mammalian spermatozoa and is required to render them competent to fertilize an oocyte. This step is a biochemical event; the sperm move normally and look mature prior to capacitation. In vivo, capacitation occurs after ejaculation, when the spermatozoa leave the vagina and enter the upper female reproductive tract. The uterus aids in the steps of capacitation by secreting sterol-binding albumin, lipoproteins, and proteolytic and glycosidasic enzymes such as heparin.

Sperm is the male reproductive cell, or gamete, in anisogamous forms of sexual reproduction. Animals produce motile sperm with a tail known as a flagellum, which are known as spermatozoa, while some red algae and fungi produce non-motile sperm cells, known as spermatia. Flowering plants contain non-motile sperm inside pollen, while some more basal plants like ferns and some gymnosperms have motile sperm.

Spermiogenesis is the final stage of spermatogenesis, during which the spermatids develop into mature spermatozoa. At the beginning of the stage, the spermatid is a more or less circular cell containing a nucleus, Golgi apparatus, centriole and mitochondria; by the end of the process, it has radically transformed into an elongated spermatozoon, complete with a head, midpiece, and tail.
Profilin is an actin-binding protein involved in the dynamic turnover and reconstruction of the actin cytoskeleton. It is found in most eukaryotic organisms. Profilin is important for spatially and temporally controlled growth of actin microfilaments, which is an essential process in cellular locomotion and cell shape changes. This restructuring of the actin cytoskeleton is essential for processes such as organ development, wound healing, and the hunting down of infectious intruders by cells of the immune system.

In biology, a protein filament is a long chain of protein monomers, such as those found in hair, muscle, or in flagella. Protein filaments form together to make the cytoskeleton of the cell. They are often bundled together to provide support, strength, and rigidity to the cell. When the filaments are packed up together, they are able to form three different cellular parts. The three major classes of protein filaments that make up the cytoskeleton include: actin filaments, microtubules and intermediate filaments.
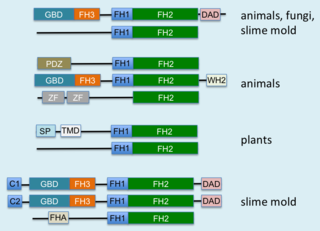
Formins (formin homology proteins) are a group of proteins that are involved in the polymerization of actin and associate with the fast-growing end (barbed end) of actin filaments. Most formins are Rho-GTPase effector proteins. Formins regulate the actin and microtubule cytoskeleton and are involved in various cellular functions such as cell polarity, cytokinesis, cell migration and SRF transcriptional activity. Formins are multidomain proteins that interact with diverse signalling molecules and cytoskeletal proteins, although some formins have been assigned functions within the nucleus.

Profilin-1 is a protein that in humans is encoded by the PFN1 gene.
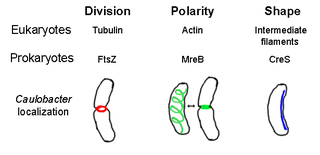
The prokaryotic cytoskeleton is the collective name for all structural filaments in prokaryotes. It was once thought that prokaryotic cells did not possess cytoskeletons, but advances in visualization technology and structure determination led to the discovery of filaments in these cells in the early 1990s. Not only have analogues for all major cytoskeletal proteins in eukaryotes been found in prokaryotes, cytoskeletal proteins with no known eukaryotic homologues have also been discovered. Cytoskeletal elements play essential roles in cell division, protection, shape determination, and polarity determination in various prokaryotes.

VEZT is a gene located on chromosome 12 and encodes for the protein vezatin. Vezatin is a major component of the cadherin-catenin complex that is critical to the formation and maintenance of adherens junctions. The protein is expressed in most epithelial cells and is crucial to the formation of cell-cell contact junctions. Mutations of the gene can lead to upregulation or downregulation of the protein which can have detrimental effects on physiological systems, particularly those involved in development.
mDia1 is a member of the protein family called the formins and is a Rho effector. It is the mouse version of the diaphanous homolog 1 of Drosophila. mDia1 localizes to cells' mitotic spindle and midbody, plays a role in stress fiber and filopodia formation, phagocytosis, activation of serum response factor, formation of adherens junctions, and it can act as a transcription factor. mDia1 accelerates actin nucleation and elongation by interacting with barbed ends of actin filaments. The gene encoding mDia1 is located on Chromosome 18 of Mus musculus and named Diap1.
Alpha-keratin, or α-keratin, is a type of keratin found in mammalian vertebrates. This protein is the primary component in hairs, horns, claws, nails and the epidermis layer of the skin. α-keratin is a fibrous structural protein, meaning it is made up of amino acids that form a repeating secondary structure. The secondary structure of α-keratin is very similar to that of a traditional protein α-helix and forms a coiled coil. Due to its tightly wound structure, it can function as one of the strongest biological materials and has various functions in mammals, from predatory claws to hair for warmth. α-keratin is synthesized through protein biosynthesis, utilizing transcription and translation, but as the cell matures and is full of α-keratin, it dies, creating a strong non-vascular unit of keratinized tissue.
References
- Kierszenbaum AL, Tres LL (November 2004). "The acrosome-acroplaxome-manchette complex and the shaping of the spermatid head". Arch. Histol. Cytol. 67 (4): 271–84. doi: 10.1679/aohc.67.271 . PMID 15700535.
- 1 2 Kierszenbaum AL, Rivkin E, Tres LL (November 2003). "Acroplaxome, an F-actin-keratin-containing plate, anchors the acrosome to the nucleus during shaping of the spermatid head". Mol. Biol. Cell. 14 (11): 4628–40. doi:10.1091/mbc.E03-04-0226. PMC 266778 . PMID 14551252.
- ↑ Obermann H, Raabe I, Balvers M, Brunswig B, Schulze W, Kirchhoff C (January 2005). "Novel testis-expressed profilin IV associated with acrosome biogenesis and spermatid elongation". Mol. Hum. Reprod. 11 (1): 53–64. doi:10.1093/molehr/gah132. PMID 15591451.