
In organic chemistry, an acyl cyanide is a functional group consisting of an acyl group (RC(O)) attached to cyanide (CN). Examples include acetyl cyanide, formyl cyanide, and oxalyl dicyanide. Acyl cyanides are reagents in organic synthesis. [1] [2]

In organic chemistry, an acyl cyanide is a functional group consisting of an acyl group (RC(O)) attached to cyanide (CN). Examples include acetyl cyanide, formyl cyanide, and oxalyl dicyanide. Acyl cyanides are reagents in organic synthesis. [1] [2]
Classically acyl cyanides are produced by the salt metathesis reaction of acyl chlorides with sodium cyanide:
Alternatively, they can be produced by dehydration of acyl aldoximes:
Acetyl cyanide is also prepared by hydrocyanation of ketene:
They are mild acylating agents. [2] With aqueous base, acyl cyanides break down to cyanide and the carboxylate: [3]
With azides, acyl cyanides undergo the click reaction to give acyl tetrazoles. [4]

Azide is the anion with the formula N−3. It is the conjugate base of hydrazoic acid HN3. N−3 is a linear anion. Organic azides are organic compounds with the formula RN3, containing the azide functional group. The dominant application of azides is as a propellant in air bags.

A Gilman reagent is a lithium and copper (diorganocopper) reagent compound, R2CuLi, where R is an alkyl or aryl. These reagents are useful because, unlike related Grignard reagents and organolithium reagents, they react with organic halides to replace the halide group with an R group (the Corey–House reaction). Such displacement reactions allow for the synthesis of complex products from simple building blocks.
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group -COCl. Their formula is usually written RCOCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

Acetyl chloride (CH3COCl) is an acyl chloride derived from acetic acid. It belongs to the class of organic compounds called acid halides. It is a colorless, corrosive, volatile liquid. Its formula is commonly abbreviated to AcCl.
An isocyanide is an organic compound with the functional group -N≡C. It is the isomer of the related nitrile (-C≡N), hence the prefix is isocyano. The organic fragment is connected to the isocyanide group through the nitrogen atom, not via the carbon. They are used as building blocks for the synthesis of other compounds.
In chemical synthesis, "click" chemistry is a class of biocompatible small molecule reactions commonly used in bioconjugation, allowing the joining of substrates of choice with specific biomolecules. Click chemistry is not a single specific reaction, but describes a way of generating products that follow examples in nature, which also generates substances by joining small modular units. In many applications, click reactions join a biomolecule and a reporter molecule. Click chemistry is not limited to biological conditions: the concept of a "click" reaction has been used in chemoproteomic, pharmacological, and various biomimetic applications. However, they have been made notably useful in the detection, localization and qualification of biomolecules.
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Karl Barry Sharpless has referred to this cycloaddition as "the cream of the crop" of click chemistry and "the premier example of a click reaction."
Tetrazoles are a class of synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen atoms and one carbon atom. The name tetrazole also refers to the parent compound with formula CH2N4, of which three isomers can be formulated.
In chemistry, a phase-transfer catalyst or PTC is a catalyst that facilitates the migration of a reactant from one phase into another phase where reaction occurs. Phase-transfer catalysis is a special form of heterogeneous catalysis. Ionic reactants are often soluble in an aqueous phase but insoluble in an organic phase in the absence of the phase-transfer catalyst. The catalyst functions like a detergent for solubilizing the salts into the organic phase. Phase-transfer catalysis refers to the acceleration of the reaction upon the addition of the phase-transfer catalyst.
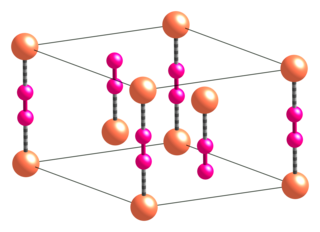
Copper(I) cyanide is an inorganic compound with the formula CuCN. This off-white solid occurs in two polymorphs; impure samples can be green due to the presence of Cu(II) impurities. The compound is useful as a catalyst, in electroplating copper, and as a reagent in the preparation of nitriles.
Umpolung or polarity inversion in organic chemistry is the chemical modification of a functional group with the aim of the reversal of polarity of that group. This modification allows secondary reactions of this functional group that would otherwise not be possible. The concept was introduced by D. Seebach and E.J. Corey. Polarity analysis during retrosynthetic analysis tells a chemist when umpolung tactics are required to synthesize a target molecule.

Iodosobenzene or iodosylbenzene is an organoiodine compound with the empirical formula C6H5IO. This colourless solid compound is used as an oxo transfer reagent in research laboratories examining organic and coordination chemistry.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. While iron adopts oxidation states from Fe(−II) through to Fe(VII), Fe(IV) is the highest established oxidation state for organoiron species. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.

Tributyltin azide is an organotin compound with the formula (C4H9)3SnN3. It is a colorless solid although older samples can appear as yellow oils. The compound is used as a reagent in organic synthesis.

Acetyl cyanide is the organic compound with the formula CH3C(O)CN. It is an acyl cyanide. Acetyl cyanide is a colorless liquid.

Cyanogen azide, N3CN or CN4, is an azide compound of carbon and nitrogen which is an oily, colourless liquid at room temperature. It is a highly explosive chemical that is soluble in most organic solvents, and normally handled in dilute solution in this form. It was first synthesised by F. D. Marsh at DuPont in the early 1960s.

1-Diazidocarbamoyl-5-azidotetrazole, often informally referred to as azidoazide azide, is a heterocyclic inorganic compound with the formula C2N14. It is an extremely sensitive explosive.
Paul Knochel is a French chemist and a member of the French Academy of Sciences.
Iodine azide is an explosive inorganic compound, which in ordinary conditions is a yellow solid. Formally, it is an inter-pseudohalogen.
An organic azide is organic compounds containing the azide (N3) functional group. Because of the hazards associated with their use, few azides are used commercially although they exhibit interesting reactivity for researchers. Low molecular weight azides are considered especially hazardous and are avoided. In the research laboratory, azides are precursors to amines. They are also popular for their participation in the "click reaction" and in Staudinger ligation. These two reactions are generally quite reliable, lending themselves to combinatorial chemistry.