In physics, a fluid is a substance that continually deforms (flows) under an applied shear stress, or external force. Fluids are a phase of matter and include liquids, gases and plasmas. They are substances with zero shear modulus, or, in simpler terms, substances which cannot resist any shear force applied to them.

In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
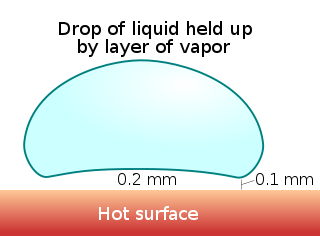
The Leidenfrost effect is a physical phenomenon in which a liquid, in near contact with a mass significantly hotter than the liquid's boiling point, produces an insulating vapor layer keeping that liquid from boiling rapidly. Because of this 'repulsive force', a droplet hovers over the surface rather than making physical contact with it. This is most commonly seen when cooking: one sprinkles drops of water in a pan to gauge its temperature: if the pan's temperature is at or above the Leidenfrost point, the water skitters across the pan and takes longer to evaporate than in a pan with a temperature below the Leidenfrost point but still above boiling. It is named after Johann Gottlob Leidenfrost, who discussed it in A Tract About Some Qualities of Common Water in 1751.
Digital microfluidics (DMF) is another platform for lab-on-a-chip systems that is based upon the manipulation of microdroplets. Droplets are dispensed, moved, stored, mixed, reacted, or analyzed on a platform with a set of insulated electrodes. Digital microfluidics can be used together with analytical analysis procedures such as mass spectrometry, colorimetry, electrochemical, and electrochemiluminescense.

A surface acoustic wave (SAW) is an acoustic wave traveling along the surface of a material exhibiting elasticity, with an amplitude that typically decays exponentially with depth into the material.
Electrowetting is the modification of the wetting properties of a surface with an applied electric field.
Smart glass or switchable glass is a glass or glazing whose light transmission properties are altered when voltage, light or heat is applied. Generally, the glass changes from translucent to transparent, changing from blocking some wavelengths of light to letting light pass through.

Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. The degree of wetting (wettability) is determined by a force balance between adhesive and cohesive forces. Wetting deals with the three phases of materials: gas, liquid, and solid. It is now a center of attention in nanotechnology and nanoscience studies due to the advent of many nanomaterials in the past two decades.

In fluid mechanics, dewetting is one of the processes that can occur at a solid–liquid or liquid–liquid interface. Generally, dewetting describes the process of retraction of a fluid from a non-wettable surface it was forced to cover. The opposite process—spreading of a liquid on a substrate—is called wetting. The factor determining the spontaneous spreading and dewetting for a drop of liquid placed on a solid substrate with ambient gas, is the so-called spreading coefficient S:
The Marangoni effect is the mass transfer along an interface between two fluids due to a gradient of the surface tension. In the case of temperature dependence, this phenomenon may be called thermo-capillary convection.

The contact angle is the angle, conventionally measured through the liquid, where a liquid–vapor interface meets a solid surface. It quantifies the wettability of a solid surface by a liquid via the Young equation. A given system of solid, liquid, and vapor at a given temperature and pressure has a unique equilibrium contact angle. However, in practice a dynamic phenomenon of contact angle hysteresis is often observed, ranging from the advancing (maximal) contact angle to the receding (minimal) contact angle. The equilibrium contact is within those values, and can be calculated from them. The equilibrium contact angle reflects the relative strength of the liquid, solid, and vapor molecular interaction.

A rheometer is a laboratory device used to measure the way in which a liquid, suspension or slurry flows in response to applied forces. It is used for those fluids which cannot be defined by a single value of viscosity and therefore require more parameters to be set and measured than is the case for a viscometer. It measures the rheology of the fluid.

Ultrahydrophobic surfaces are highly hydrophobic, i.e., extremely difficult to wet. The contact angles of a water droplet on an ultrahydrophobic material exceed 150°. This is also referred to as the lotus effect, after the superhydrophobic leaves of the lotus plant. A droplet striking these kinds of surfaces can fully rebound like an elastic ball, or pancake.
Ice protection systems are designed to keep atmospheric ice from accumulating on aircraft surfaces, such as wings, propellers, rotor blades, engine intakes, and environmental control intakes. If ice is allowed to build up to a significant thickness it can change the shape of airfoils and flight control surfaces, degrading the performance, control or handling characteristics of the aircraft. An ice protection system either prevents formation of ice, or enables the aircraft to shed the ice before it can grow to a dangerous thickness.
Stephen H. Davis is an American applied mathematician working in the fields of Fluid Mechanics and Materials Science. Davis is the McCormick School Institute Professor and the Walter P. Murphy Professor of Applied Mathematics at Northwestern University. Davis has been listed as an ISI Highly Cited researcher in Engineering.
Nuclear magnetic resonance (NMR) in porous materials covers the application of using NMR as a tool to study the structure of porous media and various processes occurring in them. This technique allows the determination of characteristics such as the porosity and pore size distribution, the permeability, the water saturation, the wettability, etc.

A fluid dynamic gauge (FDG) is a measurement technique used to study the behaviour of soft deposit layers in a liquid environment. It employs fluid mechanics to determine the thickness of the layer, and can also be used to obtain a measure of its strength. It was inspired by the technique of pneumatic gauging, which relies on a flow of air rather than the process liquid. Fluid dynamic gauging can be conducted as an in-line measuring technique, but is more commonly used as a research tool.

Elasto-capillarity is the ability of capillary force to deform an elastic material. From the viewpoint of mechanics, elastocapillarity phenomena essentially involve competition between the elastic strain energy in the bulk and the energy on the surfaces/interfaces. In the modeling of these phenomena, some challenging issues are, among others, the exact characterization of energies at the micro scale, the solution of strongly nonlinear problems of structures with large deformation and moving boundary conditions, and instability of either solid structures or droplets/films.The capillary forces are generally negligible in the analysis of macroscopic structures but often play a significant role in many phenomena at small scales.

Drop impact occurs when a liquid drop strikes a solid or liquid surface. The resulting outcome depends on the properties of the drop, the surface, and the surrounding fluid, which is most commonly a gas.
Self-cleaning surfaces are a class of materials with the inherent ability to remove any debris or bacteria from their surfaces in a variety of ways. The self-cleaning functionality of these surfaces are commonly inspired by natural phenomena observed in lotus leaves, gecko feet, and water striders to name a few. The majority of self-cleaning surfaces can be placed into three categories: 1) Superhydrophobic, 2) Superhydrophilic, and 3) Photocatalytic.












