
Clathrin is a protein that plays a major role in the formation of coated vesicles. Clathrin was first isolated and named by Barbara Pearse in 1976. It forms a triskelion shape composed of three clathrin heavy chains and three light chains. When the triskelia interact they form a polyhedral lattice that surrounds the vesicle, hence the protein's name, which is derived from the Latin clathrum meaning lattice. Coat-proteins, like clathrin, are used to build small vesicles in order to transport molecules within cells. The endocytosis and exocytosis of vesicles allows cells to communicate, to transfer nutrients, to import signaling receptors, to mediate an immune response after sampling the extracellular world, and to clean up the cell debris left by tissue inflammation. The endocytic pathway can be hijacked by viruses and other pathogens in order to gain entry to the cell during infection.
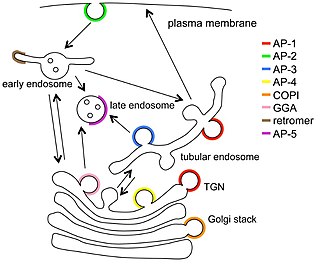
Vesicular transport adaptor proteins are proteins involved in forming complexes that function in the trafficking of molecules from one subcellular location to another. These complexes concentrate the correct cargo molecules in vesicles that bud or extrude off of one organelle and travel to another location, where the cargo is delivered. While some of the details of how these adaptor proteins achieve their trafficking specificity has been worked out, there is still much to be learned.

The AP2 adaptor complex is a multimeric protein that works on the cell membrane to internalize cargo in clathrin-mediated endocytosis. It is a stable complex of four adaptins which give rise to a structure that has a core domain and two appendage domains attached to the core domain by polypeptide linkers. These appendage domains are sometimes called 'ears'. The core domain binds to the membrane and to cargo destined for internalisation. The alpha and beta appendage domains bind to accessory proteins and to clathrin. Their interactions allow the temporal and spatial regulation of the assembly of clathrin-coated vesicles and their endocytosis.

AP-2 complex subunit mu is a protein that in humans is encoded by the AP2M1 gene.

AP-2 complex subunit alpha-1 is a protein that in humans is encoded by the AP2A1 gene.

AP-1 complex subunit mu-1 is a protein that in humans is encoded by the AP1M1 gene.

AP-1 complex subunit gamma-1 is a protein that in humans is encoded by the AP1G1 gene.

AP-1 complex subunit beta-1 is a protein that in humans is encoded by the AP1B1 gene.

AP-2 complex subunit beta is a protein that in humans is encoded by the AP2B1 gene.

AP-1 complex subunit mu-2 is a protein that in humans is encoded by the AP1M2 gene.

AP-1 complex subunit sigma-1A is a protein that in humans is encoded by the AP1S1 gene.

AP-1 complex subunit gamma-like 2 is a protein that in humans is encoded by the AP1G2 gene.

AP-3 complex subunit mu-1 is a protein that in humans is encoded by the AP3M1 gene.

AP-1 complex subunit sigma-2 is a protein that in humans is encoded by the AP1S2 gene.

AP-2 complex subunit sigma is a protein that in humans is encoded by the AP2S1 gene.

AP-4 complex subunit beta-1 is a protein that in humans is encoded by the AP4B1 gene.

AP-4 complex subunit mu-1 is a protein that in humans is encoded by the AP4M1 gene.

AP-4 complex subunit epsilon-1 is a protein that in humans is encoded by the AP4E1 gene.
Clathrin adaptor proteins, also known as adaptins, are vesicular transport adaptor proteins associated with clathrin. These proteins are synthesized in the ribosomes, processed in the endoplasmic reticulum and transported from the Golgi apparatus to the trans-Golgi network, and from there via small carrier vesicles to their final destination compartment. The association between adaptins and clathrin are important for vesicular cargo selection and transporting. Clathrin coats contain both clathrin and adaptor complexes that link clathrin to receptors in coated vesicles. Clathrin-associated protein complexes are believed to interact with the cytoplasmic tails of membrane proteins, leading to their selection and concentration. Therefore, adaptor proteins are responsible for the recruitment of cargo molecules into a growing clathrin-coated pits. The two major types of clathrin adaptor complexes are the heterotetrameric vesicular transport adaptor proteins (AP1-5), and the monomeric GGA adaptors. Adaptins are distantly related to the other main type of vesicular transport proteins, the coatomer subunits, sharing between 16% and 26% of their amino acid sequence.

The C-terminal domain ofBeta2-adaptin is a protein domain is involved in cell trafficking by aiding import and export of substances in and out of the cell.

















