Related Research Articles

A randomized controlled trial is a type of scientific experiment that aims to reduce certain sources of bias when testing the effectiveness of new treatments; this is accomplished by randomly allocating subjects to two or more groups, treating them differently, and then comparing them with respect to a measured response. One group—the experimental group—receives the intervention being assessed, while the other—usually called the control group—receives an alternative treatment, such as a placebo or no intervention. The groups are monitored under conditions of the trial design to determine the effectiveness of the experimental intervention, and efficacy is assessed in comparison to the control. There may be more than one treatment group or more than one control group.
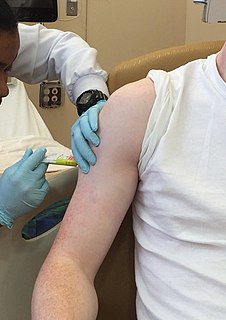
Clinical trials are experiments or observations done in clinical research. Such prospective biomedical or behavioral research studies on human participants are designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.

Rofecoxib is a COX-2 selective nonsteroidal anti-inflammatory drug (NSAID). It was marketed by Merck & Co. to treat osteoarthritis, rheumatoid arthritis, juvenile rheumatoid arthritis, acute pain conditions, migraine, and dysmenorrhea. Rofecoxib was approved in the US by the US Food and Drug Administration (FDA) in May 1999, and was marketed under the brand names Vioxx, Ceoxx, and Ceeoxx. Rofecoxib was available by prescription in both tablet-form and as an oral suspension.

Asherman's syndrome (AS), is an acquired uterine condition that occurs when scar tissue (adhesions) form inside the uterus and/or the cervix. It is characterized by variable scarring inside the uterine cavity, where in many cases the front and back walls of the uterus stick to one another. AS can be the cause of menstrual disturbances, infertility, and placental abnormalities. Although the first case of intrauterine adhesion was published in 1894 by Heinrich Fritsch, it was only after 54 years that a full description of Asherman syndrome was carried out by Joseph Asherman. A number of other terms have been used to describe the condition and related conditions including: uterine/cervical atresia, traumatic uterine atrophy, sclerotic endometrium, and endometrial sclerosis.

Desonide (INN) is a low-potency topical corticosteroid anti-inflammatory that has been available since the 1970s. It is primarily used to treat atopic dermatitis (eczema), seborrheic dermatitis, contact dermatitis and psoriasis in both adults and children. It has a fairly good safety profile and is available as a cream, ointment, lotion, and as a foam under the tradename Verdeso Foam. Other trade names for creams, lotions, and ointments include Tridesilon, DesOwen, Desonate. It is a group VI corticosteroid under US classification, the second least potent group.

A breast implant is a prosthesis used to change the size, shape, and contour of a person's breast. In reconstructive plastic surgery, breast implants can be placed to restore a natural looking breast following a mastectomy or to correct congenital defects and deformities of the chest wall. They are also used cosmetically to enlarge the appearance of the breast through breast augmentation surgery.

A drug-eluting stent (DES) is a peripheral or coronary stent placed into narrowed, diseased peripheral or coronary arteries that slowly releases a drug to block cell proliferation. This prevents fibrosis that, together with clots (thrombi), could otherwise block the stented artery, a process called restenosis. The stent is usually placed within the peripheral or coronary artery by an interventional cardiologist or interventional radiologist during an angioplasty procedure.

Glatiramer acetate is an immunomodulator medication currently used to treat multiple sclerosis. Glatiramer acetate is approved in the United States to reduce the frequency of relapses, but not for reducing the progression of disability. Observational studies, but not randomized controlled trials, suggest that it may reduce progression of disability. While a conclusive diagnosis of multiple sclerosis requires a history of two or more episodes of symptoms and signs, glatiramer acetate is approved to treat a first episode anticipating a diagnosis. It is also used to treat relapsing-remitting multiple sclerosis. It is administered by subcutaneous injection.
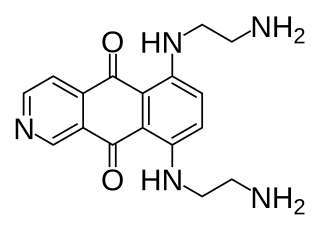
Pixantrone is an experimental antineoplastic (anti-cancer) drug, an analogue of mitoxantrone with fewer toxic effects on cardiac tissue. It acts as a topoisomerase II poison and intercalating agent. The code name BBR 2778 refers to pixantrone dimaleate, the actual substance commonly used in clinical trials.
An adhesion barrier is a medical implant that can be used to reduce abnormal internal scarring (adhesions) following surgery by separating the internal tissues and organs while they heal.
A glossary of terms used in clinical research.
The following outline is provided as an overview of and topical guide to clinical research:

Olaparib, sold under the brand name Lynparza, is a medication for the maintenance treatment of BRCA-mutated advanced ovarian cancer in adults. It is a PARP inhibitor, inhibiting poly ADP ribose polymerase (PARP), an enzyme involved in DNA repair. It acts against cancers in people with hereditary BRCA1 or BRCA2 mutations, which include some ovarian, breast, and prostate cancers.

Genta Incorporated was a biopharmaceutical company started in La Jolla, California, which discovered and developed innovative drugs for the treatment of patients with cancer. Founded in 1989 by a highly skilled entrepreneur, the company focused on a novel technology known as antisense, which targets gene products that are associated with the onset and progression of serious diseases. At that time, only Ionis Pharmaceuticals, Inc. was conducting significant research with this technology. Antisense is a short span of oligonucleotides – modified DNA structures ranging from about 12-24 bases that selectively bind to specific RNA. The intent is to block expression of an aberrant protein that contributes to the disease of interest. Genta in-licensed three different antisense molecules that blocked Bcl-2, a fibroblast growth factor (FGF), and the gene c-myb, respectively.
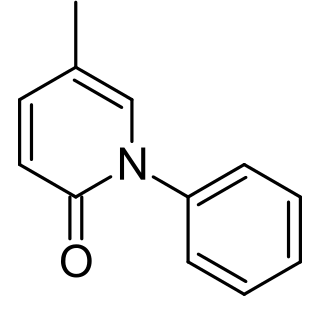
Pirfenidone is a medication used for the treatment of idiopathic pulmonary fibrosis. It works by reducing lung fibrosis through downregulation of the production of growth factors and procollagens I and II.
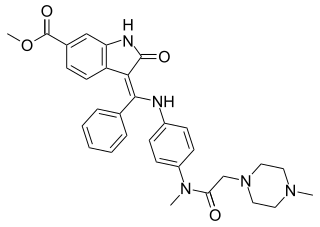
Nintedanib, sold under the brand names Ofev and Vargatef, is an oral medication used for the treatment of idiopathic pulmonary fibrosis and along with other medications for some types of non-small-cell lung cancer.
Ixekizumab, sold under the brand name Taltz, is an injectable medication for the treatment of autoimmune diseases. Chemically, it is a form of a humanized monoclonal antibody. The substance acts by binding interleukin 17A and neutralizing it, reducing inflammation.
Atezolizumab, sold under the brand name Tecentriq, is a monoclonal antibody medication used to treat urothelial carcinoma, non-small cell lung cancer (NSCLC), triple-negative breast cancer (TNBC), small cell lung cancer (SCLC), and hepatocellular carcinoma (HCC). It is a fully humanized, engineered monoclonal antibody of IgG1 isotype against the protein programmed cell death-ligand 1 (PD-L1).

Calcipotriol/betamethasone dipropionate, sold under the brand name Taclonex among others, is a fixed-dose combination medication of the synthetic vitamin D3 analog calcipotriol (also known as calcipotriene) and the synthetic corticosteroid betamethasone dipropionate for the treatment of plaque psoriasis. It is used in the form of ointment, topical suspension, gel, aerosol, and foam.
Risankizumab, sold under the brand name Skyrizi, is a humanized monoclonal antibody targeting interleukin 23A (IL-23A). Risankizumab is part of a collaboration between Boehringer Ingelheim and AbbVie. Risankizumab has been approved in the European Union, the United States, Canada, and Japan for treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.
References
- ↑ International Adhesions Society. Products Approved by FDA for Adhesion Prevention, Reduction in Pelvice and/or Abdominal Cavities. (n.d.). Retrieved April 17, 2016, from http://www.adhesions.org/products.htm
- 1 2 Larking, P., & Dwairy, M. (2005). Adcon-L Effectiveness Preventing Peridural Fibrosis. Retrieved April 17, 2016.
| This dermatologic drug article is a stub. You can help Wikipedia by expanding it. |