Related Research Articles
Adrian Vivian Sinton Hill, is an Irish vaccinologist, Director of the Jenner Institute and Lakshmi Mittal and Family Professor of Vaccinology at the University of Oxford, an honorary Consultant Physician in Infectious Diseases, and Fellow of Magdalen College, Oxford. Hill is a leader in the field of malaria vaccine development and was a co-leader of the research team which produced the Oxford–AstraZeneca COVID-19 vaccine, along with Professor Sarah Gilbert of the Jenner Institute and Professor Andrew Pollard of the Oxford Vaccine Group.

Helen Rees OBE GCOB is a medical researcher and the founder and Executive Director of the Wits Reproductive Health and HIV Institute of the University of Witwatersrand. She has led many HIV prevention and sexual and reproductive health studies and advised on vaccination strategies to help prevent various medical conditions.

Chikwe Ihekweazu is a Nigerian epidemiologist and public health physician who has been serving as the World Health Organization’s assistant director-general of health emergency intelligence since 2021.
Dame Sarah Catherine Gilbert is a British vaccinologist who is Saïd Professor of Vaccinology at the University of Oxford and co-founder of Vaccitech. Gilbert specialises in the development of vaccines against influenza and emerging viral pathogens. She led the development and testing of the universal flu vaccine, which underwent clinical trials in 2011. On New Year's Day 2020 Gilbert read on ProMED-mail about four people in China suffering from a strange pneumonia of unknown cause, in Wuhan, China. Within two weeks a vaccine had been designed at Oxford against the new pathogen. On 30 December 2020, the Oxford–AstraZeneca COVID-19 vaccine she co-developed with the Oxford Vaccine Group was approved for use in the United Kingdom. By July 2021, one billion doses of the vaccine had been released to more than 170 countries worldwide.

Jonathan Stafford Nguyen Van-Tam is a British healthcare professional specialising in influenza, including its epidemiology, transmission, vaccinology, antiviral drugs and pandemic preparedness.

AD5-nCOV, trade-named Convidecia, is a single-dose viral vector vaccine for COVID-19 developed by CanSino Biologics. It conducted its Phase III trials in Argentina, Chile, Mexico, Pakistan, Russia, and Saudi Arabia with 40,000 participants.

The Africa Centres for Disease Control and Prevention is a public health agency of the African Union to support the public health initiatives of member states and strengthen the capacity of their health institutions to deal with disease threats. The Africa CDC ideas was proposed by the government of Ethiopia in 2013 during TB/HIV special submit in Abuja Nigeria. From 2013 to 2016 the modalities and statue of Africa CDC were developed and the specialized agency was officially launched in January 2017.

The Oxford–AstraZeneca COVID-19 vaccine, codenamed AZD1222, and sold under the brand names Covishield and Vaxzevria among others, is a viral vector vaccine for prevention of COVID-19. Developed in the United Kingdom by the Oxford University and British-Swedish company AstraZeneca, it is given by intramuscular injection, using as a vector the modified chimpanzee adenovirus ChAdOx1. Studies carried out in 2020 showed that the efficacy of the vaccine is 76.0% at preventing symptomatic COVID-19 beginning at 22 days following the first dose and 81.3% after the second dose. A study in Scotland found that, for symptomatic COVID-19 infection after the second dose, the vaccine is 81% effective against the Alpha variant, and 61% against the Delta variant.
Sir Andrew John Pollard is a Professor of Paediatric Infection and Immunity at the University of Oxford and a Fellow of St Cross College, Oxford. He is an Honorary Consultant Paediatrician at John Radcliffe Hospital and the Director of the Oxford Vaccine Group. He is the Chief Investigator on the University of Oxford COVID-19 Vaccine trials and has led research on vaccines for many life-threatening infectious diseases including typhoid fever, Neisseria meningitidis, Haemophilus influenzae type b, streptococcus pneumoniae, pertussis, influenza, rabies, and Ebola.

The Novavax COVID-19 vaccine, codenamed NVX-CoV2373, is a subunit COVID-19 vaccine candidate developed by Novavax and the Coalition for Epidemic Preparedness Innovations (CEPI), that is undergoing trials in India under the brand name Covovax. It requires two doses and is stable at 2 to 8 °C refrigerated temperatures.

Beta variant, also known as lineage B.1.351, is a variant of SARS-CoV-2, the virus that causes COVID-19. One of several SARS-CoV-2 variants believed to be of particular importance, it was first detected in the Nelson Mandela Bay metropolitan area of the Eastern Cape province of South Africa in October 2020, which was reported by the country's health department on 18 December 2020. Phylogeographic analysis suggests this variant emerged in the Nelson Mandela Bay area in July or August 2020.
The following is a timeline of the COVID-19 pandemic in England during 2021. There are significant differences in the legislation and the reporting between the countries of the UK: England, Scotland, Northern Ireland, and Wales.

Shabir Ahmed Madhi is a South African physician who is professor of vaccinology and director of the South African Medical Research Council Respiratory and Meningeal Pathogens Research Unit at the University of the Witwatersrand, and National Research Foundation/Department of Science and Technology Research Chair in Vaccine Preventable Diseases. In January 2021, he was appointed Dean of the Faculty of Health Sciences at the University of the Witwateratand.
The National Advisory Group on Immunization (NAGI), in South Africa, established in 1993 advises the National Department of Health on issues pertinent to vaccination and infectious diseases. It makes recommendations on vaccine formulations and vaccination schedules.

Zhang Yongzhen, also known as Yong-Zhen Zhang, is a Chinese virologist known for his work relating to the COVID-19 pandemic. A professor at Fudan University, Zhang has discovered numerous RNA viruses and created a network of labs dedicated to monitoring new viruses. He led the team that sequenced and published the genome of SARS-CoV-2, the virus that causes COVID-19, in early January 2020.

COVID-19 vaccination in South Africa is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.
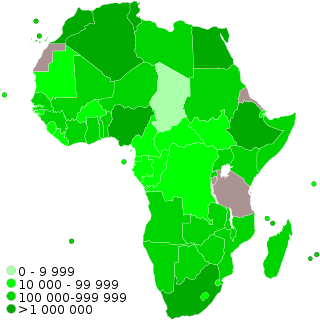
COVID-19 vaccination programs have begun in many countries and territories in Africa. As of 5 July 2021, vaccination campaigns had started in 51 African countries with 36.5 million people receiving at least one dose.

The South African Health Products Regulatory Authority (SAHPRA) is the organization in charge of regulating the use of all Health Products throughout the country.
Eleanor Barnes is a British physician at the John Radcliffe Hospital and a Professor of Hepatology and Experimental Medicine at the University of Oxford. She has studied hepatitis C and the development of the development of HCV vaccines. She is a Fellow of the Academy of Medical Sciences.
References
- 1 2 "Home". www.witsalive.co.za. Retrieved 8 January 2021.
- ↑ Moïsi, Jennifer; Madhi, Shabir Ahmed; Rees, Helen (2019). "Vaccinology in sub-Saharan Africa". BMJ Global Health. 4 (5): e001363. doi: 10.1136/bmjgh-2018-001363 . ISSN 2059-7908. PMC 6768329 . PMID 31637022.
- ↑ "Imprint Network - New Masters Programme in Vaccinology". www.imprint-network.co.uk. Retrieved 8 January 2021.
- ↑ "Immunization, Vaccines and Biologicals: Professor Shabir A. Madhi". World Health Organization. Retrieved 8 January 2021.