
An anticoagulant, commonly known as a blood thinner, is a chemical substance that prevents or reduces the coagulation of blood, prolonging the clotting time. Some occur naturally in blood-eating animals, such as leeches and mosquitoes, which help keep the bite area unclotted long enough for the animal to obtain blood.
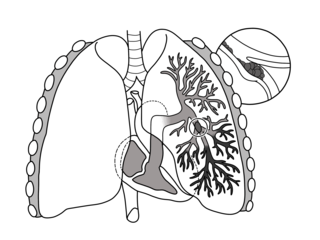
Pulmonary embolism (PE) is a blockage of an artery in the lungs by a substance that has moved from elsewhere in the body through the bloodstream (embolism). Symptoms of a PE may include shortness of breath, chest pain particularly upon breathing in, and coughing up blood. Symptoms of a blood clot in the leg may also be present, such as a red, warm, swollen, and painful leg. Signs of a PE include low blood oxygen levels, rapid breathing, rapid heart rate, and sometimes a mild fever. Severe cases can lead to passing out, abnormally low blood pressure, obstructive shock, and sudden death.

Venous thrombosis is the blockage of a vein caused by a thrombus. A common form of venous thrombosis is deep vein thrombosis (DVT), when a blood clot forms in the deep veins. If a thrombus breaks off (embolizes) and flows to the lungs to lodge there, it becomes a pulmonary embolism (PE), a blood clot in the lungs. The conditions of DVT only, DVT with PE, and PE only, are all captured by the term venous thromboembolism (VTE).

Deep vein thrombosis (DVT) is a type of venous thrombosis involving the formation of a blood clot in a deep vein, most commonly in the legs or pelvis. A minority of DVTs occur in the arms. Symptoms can include pain, swelling, redness, and enlarged veins in the affected area, but some DVTs have no symptoms.
Low-molecular-weight heparin (LMWH) is a class of anticoagulant medications. They are used in the prevention of blood clots and, in the treatment of venous thromboembolism, and the treatment of myocardial infarction.
D-dimer is a dimer that is a fibrin degradation product (FDP), a small protein fragment present in the blood after a blood clot is degraded by fibrinolysis. It is so named because it contains two D fragments of the fibrin protein joined by a cross-link, hence forming a protein dimer.
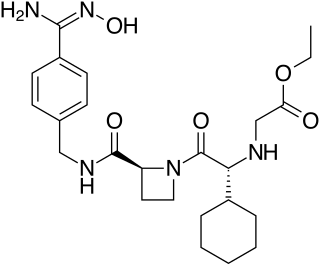
Ximelagatran is an anticoagulant that has been investigated extensively as a replacement for warfarin that would overcome the problematic dietary, drug interaction, and monitoring issues associated with warfarin therapy. In 2006, its manufacturer AstraZeneca announced that it would withdraw pending applications for marketing approval after reports of hepatotoxicity during trials, and discontinue its distribution in countries where the drug had been approved.

Factor XI, or plasma thromboplastin antecedent, is the zymogen form of factor XIa, one of the enzymes involved in coagulation. Like many other coagulation factors, it is a serine protease. In humans, factor XI is encoded by F11 gene.
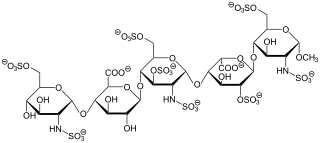
Fondaparinux is an anticoagulant medication chemically related to low molecular weight heparins. It is marketed by Viatris. A generic version developed by Alchemia is marketed within the US by Dr. Reddy's Laboratories.

Rivaroxaban, sold under the brand name Xarelto among others, is an anticoagulant medication used to treat and prevent blood clots. Specifically it is used to treat deep vein thrombosis and pulmonary emboli and prevent blood clots in atrial fibrillation and following hip or knee surgery. It is taken by mouth.
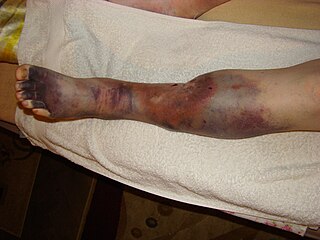
Post-thrombotic syndrome (PTS), also called postphlebitic syndrome and venous stress disorder is a medical condition that may occur as a long-term complication of deep vein thrombosis (DVT).
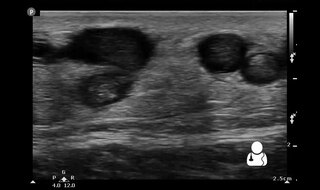
Superficial thrombophlebitis is a thrombosis and inflammation of superficial veins presenting as a painful induration (thickening) with erythema, often in a linear or branching configuration with a cordlike appearance.
Betrixaban is an oral anticoagulant drug which acts as a direct factor Xa inhibitor. Betrixaban is FDA approved for venous thrombosis prevention in adults hospitalized for an acute illness who are at risk for thromboembolic complications. Compared to other directly acting oral anticoagulants betrixaban has relatively low renal excretion and is not metabolized by CYP3A4.

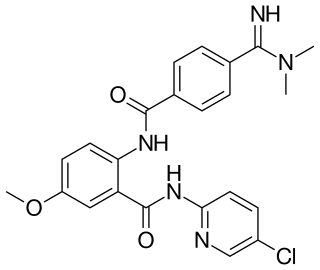
Darexaban (YM150) is a direct inhibitor of factor Xa created by Astellas Pharma. It is an experimental drug that acts as an anticoagulant and antithrombotic to prevent venous thromboembolism after a major orthopaedic surgery, stroke in patients with atrial fibrillation and possibly ischemic events in acute coronary syndrome. It is used in form of the maleate. The development of darexaban was discontinued in September 2011.
Andexanet alfa, sold under the brand name Andexxa among others, is an antidote for the medications rivaroxaban and apixaban, when reversal of anticoagulation is needed due to uncontrolled bleeding. It has not been found to be useful for other factor Xa inhibitors. It is given by injection into a vein.

Thrombosis prevention or thromboprophylaxis is medical treatment to prevent the development of thrombosis in those considered at risk for developing thrombosis. Some people are at a higher risk for the formation of blood clots than others, such as those with cancer undergoing a surgical procedure. Prevention measures or interventions are usually begun after surgery as the associated immobility will increase a person's risk.
Four drugs from the class of direct Xa inhibitors are marketed worldwide. Rivaroxaban (Xarelto) was the first approved FXa inhibitor to become commercially available in Europe and Canada in 2008. The second one was apixaban (Eliquis), approved in Europe in 2011 and in the United States in 2012. The third one edoxaban was approved in Japan in 2011 and in Europe and the US in 2015. Betrixaban (Bevyxxa) was approved in the US in 2017.
Henri Bounameaux is a clinical faculty and Professor of Medicine (hon), specialized in internal and vascular medicine (angiology), and general medicine.

Milvexian is a factor XIa inhibitor which acts as an anticoagulant. It is taken by mouth. As of late 2021, it was under study for the prevention of blood clots in patients undergoing surgery. In 2018–2023, Bristol-Myers Squibb studied milvexian for the prevention of stroke.

Marie Claire McLintock was a New Zealand haematologist and obstetric physician. She was an expert in medical conditions and disorders related to bleeding and blood clotting, and medical problems associated with pregnancy.













