Related Research Articles

An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit. Electrodes are essential parts of batteries that can consist of a variety of materials depending on the type of battery.

A lithium-ion or Li-ion battery is a type of rechargeable battery that uses the reversible intercalation of Li+ ions into electronically conducting solids to store energy. In comparison with other commercial rechargeable batteries, Li-ion batteries are characterized by higher specific energy, higher energy density, higher energy efficiency, a longer cycle life, and a longer calendar life. Also noteworthy is a dramatic improvement in lithium-ion battery properties after their market introduction in 1991: within the next 30 years, their volumetric energy density increased threefold while their cost dropped tenfold.

John Bannister Goodenough was an American materials scientist, a solid-state physicist, and a Nobel laureate in chemistry. From 1986 he was a professor of Materials Science, Electrical Engineering and Mechanical Engineering, at the University of Texas at Austin. He is credited with identifying the Goodenough–Kanamori rules of the sign of the magnetic superexchange in materials, with developing materials for computer random-access memory and with inventing cathode materials for lithium-ion batteries.
The electrochemical window (EW) of a substance is the electrode electric potential range between which the substance is neither oxidized nor reduced. The EW is one of the most important characteristics to be identified for solvents and electrolytes used in electrochemical applications. The EW is a term that is commonly used to indicate the potential range and the potential difference. It is calculated by subtracting the reduction potential from the oxidation potential.

Sir Michael Stanley Whittingham is a British-American chemist. He is a professor of chemistry and director of both the Institute for Materials Research and the Materials Science and Engineering program at Binghamton University, State University of New York. He also serves as director of the Northeastern Center for Chemical Energy Storage (NECCES) of the U.S. Department of Energy at Binghamton. He was awarded the Nobel Prize in Chemistry in 2019 alongside Akira Yoshino and John B. Goodenough.

The lithium iron phosphate battery or LFP battery is a type of lithium-ion battery using lithium iron phosphate as the cathode material, and a graphitic carbon electrode with a metallic backing as the anode. Because of their low cost, high safety, low toxicity, long cycle life and other factors, LFP batteries are finding a number of roles in vehicle use, utility-scale stationary applications, and backup power. LFP batteries are cobalt-free. As of September 2022, LFP type battery market share for EVs reached 31%, and of that, 68% were from EV makers Tesla and BYD alone. Chinese manufacturers currently hold a near monopoly of LFP battery type production. With patents having started to expire in 2022 and the increased demand for cheaper EV batteries, LFP type production is expected to rise further and surpass lithium nickel manganese cobalt oxides (NMC) type batteries in 2028.

Lithium cobalt oxide, sometimes called lithium cobaltate or lithium cobaltite, is a chemical compound with formula LiCoO
2. The cobalt atoms are formally in the +3 oxidation state, hence the IUPAC name lithium cobalt(III) oxide.

A solid-state battery is an electrical battery that uses a solid electrolyte for ionic conductions between the electrodes, instead of the liquid or gel polymer electrolytes found in conventional batteries. Solid-state batteries theoretically offer much higher energy density than the typical lithium-ion or lithium polymer batteries.
The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.
A metal–air electrochemical cell is an electrochemical cell that uses an anode made from pure metal and an external cathode of ambient air, typically with an aqueous or aprotic electrolyte.
A lithium ion manganese oxide battery (LMO) is a lithium-ion cell that uses manganese dioxide, MnO
2, as the cathode material. They function through the same intercalation/de-intercalation mechanism as other commercialized secondary battery technologies, such as LiCoO
2. Cathodes based on manganese-oxide components are earth-abundant, inexpensive, non-toxic, and provide better thermal stability.
Research in lithium-ion batteries has produced many proposed refinements of lithium-ion batteries. Areas of research interest have focused on improving energy density, safety, rate capability, cycle durability, flexibility, and reducing cost.

NASICON is an acronym for sodium (Na) super ionic conductor, which usually refers to a family of solids with the chemical formula Na1+xZr2SixP3−xO12, 0 < x < 3. In a broader sense, it is also used for similar compounds where Na, Zr and/or Si are replaced by isovalent elements. NASICON compounds have high ionic conductivities, on the order of 10−3 S/cm, which rival those of liquid electrolytes. They are caused by hopping of Na ions among interstitial sites of the NASICON crystal lattice.

Jean-Marie Tarascon FRSC is Professor of Chemistry at the Collège de France in Paris and Director of the French Research Network on Electrochemical Energy Storage (RS2E).
Lithium hybrid organic batteries are an energy storage device that combines lithium with an organic polymer. For example, polyaniline vanadium (V) oxide (PAni/V2O5) can be incorporated into the nitroxide-polymer lithium iron phosphate battery, PTMA/LiFePO4. Together, they improve the lithium ion intercalation capacity, cycle life, electrochemical performances, and conductivity of batteries.
Yang Shao-Horn is a Chinese American scholar, Professor of Mechanical Engineering and Materials Science and Engineering and a member of Research Laboratory of Electronics at the Massachusetts Institute of Technology. She is known for research on understanding and controlling of processes for storing electrons in chemical bonds towards zero-carbon energy and chemicals.
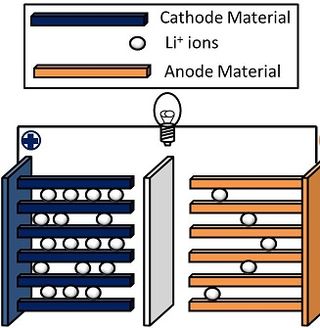
Lithium nickel manganese cobalt oxides (abbreviated NMC, Li-NMC, LNMC, or NCM) are mixed metal oxides of lithium, nickel, manganese and cobalt with the general formula LiNixMnyCo1-x-yO2. These materials are commonly used in lithium-ion batteries for mobile devices and electric vehicles, acting as the positively charged cathode.
Arumugam Manthiram is an Indian-American materials scientist and engineer, best known for his identification of the polyanion class of lithium ion battery cathodes, understanding of how chemical instability limits the capacity of layered oxide cathodes, and technological advances in lithium sulfur batteries. He is a Cockrell Family Regents Chair in engineering, Director of the Texas Materials Institute, the Director of the Materials Science and Engineering Program at the University of Texas at Austin, and a former lecturer of Madurai Kamaraj University. Manthiram delivered the 2019 Nobel Lecture in Chemistry on behalf of Chemistry Laureate John B. Goodenough.

A solid-state electrolyte (SSE) is a solid ionic conductor and electron-insulating material and it is the characteristic component of the solid-state battery. It is useful for applications in electrical energy storage (EES) in substitution of the liquid electrolytes found in particular in lithium-ion battery. The main advantages are the absolute safety, no issues of leakages of toxic organic solvents, low flammability, non-volatility, mechanical and thermal stability, easy processability, low self-discharge, higher achievable power density and cyclability. This makes possible, for example, the use of a lithium metal anode in a practical device, without the intrinsic limitations of a liquid electrolyte thanks to the property of lithium dendrite suppression in the presence of a solid-state electrolyte membrane. The use of a high capacity anode and low reduction potential, like lithium with a specific capacity of 3860 mAh g−1 and a reduction potential of -3.04 V vs SHE, in substitution of the traditional low capacity graphite, which exhibits a theoretical capacity of 372 mAh g−1 in its fully lithiated state of LiC6, is the first step in the realization of a lighter, thinner and cheaper rechargeable battery. Moreover, this allows the reach of gravimetric and volumetric energy densities, high enough to achieve 500 miles per single charge in an electric vehicle. Despite the promising advantages, there are still many limitations that are hindering the transition of SSEs from academia research to large-scale production, depending mainly on the poor ionic conductivity compared to that of liquid counterparts. However, many car OEMs (Toyota, BMW, Honda, Hyundai) expect to integrate these systems into viable devices and to commercialize solid-state battery-based electric vehicles by 2025.

This is a history of the lithium-ion battery.
References
- ↑ Rougier, Aline (January 1, 1995). Relation entre la structure et le comportement électrochimique des phases LixNi1-yMyO2(M=Al, Fe, Co) : matériaux d'électrodes positives pour batteries au lithium (These de doctorat). Bordeaux 1 – via theses.fr.
- 1 2 "Editorial Board – Solar Energy Materials & Solar Cells". Elsevier.
- ↑ "ROUGIER Aline CV" (PDF). jnoejc2019.sciencesconf.org. 2019.
- ↑ "Former staff and alumni – Aline Rougier – Laboratoire de Réactivité et Chimie des Solides". www.lrcs.u-picardie.fr.
- ↑ "Highlights – Chemistry and Electroceramics". ICMCB.