
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters.

The adrenergic receptors or adrenoceptors are a class of G protein-coupled receptors that are targets of many catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) produced by the body, but also many medications like beta blockers, beta-2 (β2) agonists and alpha-2 (α2) agonists, which are used to treat high blood pressure and asthma, for example.

Systole is the part of the cardiac cycle during which some chambers of the heart contract after refilling with blood.

Smooth muscle is an involuntary non-striated muscle, so-called because it has no sarcomeres and therefore no striations. It is divided into two subgroups, single-unit and multiunit smooth muscle. Within single-unit muscle, the whole bundle or sheet of smooth muscle cells contracts as a syncytium.

Vasoconstriction is the narrowing of the blood vessels resulting from contraction of the muscular wall of the vessels, in particular the large arteries and small arterioles. The process is the opposite of vasodilation, the widening of blood vessels. The process is particularly important in controlling hemorrhage and reducing acute blood loss. When blood vessels constrict, the flow of blood is restricted or decreased, thus retaining body heat or increasing vascular resistance. This makes the skin turn paler because less blood reaches the surface, reducing the radiation of heat. On a larger level, vasoconstriction is one mechanism by which the body regulates and maintains mean arterial pressure.

Vasodilation, also known as vasorelaxation, is the widening of blood vessels. It results from relaxation of smooth muscle cells within the vessel walls, in particular in the large veins, large arteries, and smaller arterioles. The process is the opposite of vasoconstriction, which is the narrowing of blood vessels.
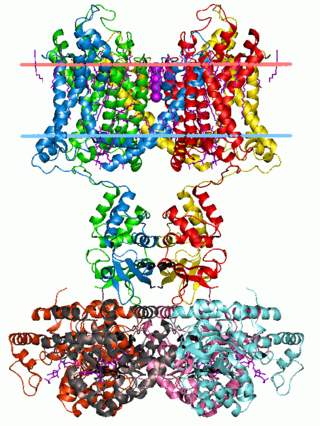
Potassium channels are the most widely distributed type of ion channel found in virtually all organisms. They form potassium-selective pores that span cell membranes. Potassium channels are found in most cell types and control a wide variety of cell functions.
Vasospasm refers to a condition in which an arterial spasm leads to vasoconstriction. This can lead to tissue ischemia and tissue death (necrosis). Cerebral vasospasm may arise in the context of subarachnoid hemorrhage. Symptomatic vasospasm or delayed cerebral ischemia is a major contributor to post-operative stroke and death especially after aneurysmal subarachnoid hemorrhage. Vasospasm typically appears 4 to 10 days after subarachnoid hemorrhage.
Hypoxic pulmonary vasoconstriction (HPV), also known as the Euler-Liljestrand mechanism, is a physiological phenomenon in which small pulmonary arteries constrict in the presence of alveolar hypoxia. By redirecting blood flow from poorly-ventilated lung regions to well-ventilated lung regions, HPV is thought to be the primary mechanism underlying ventilation/perfusion matching.
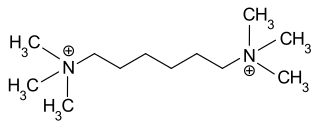
Hexamethonium is a non-depolarising ganglionic blocker, a neuronal nicotinic (nAChR) receptor antagonist that acts in autonomic ganglia by binding mostly in or on the nAChR receptor, and not the acetylcholine binding site itself. It does not have any effect on the muscarinic acetylcholine receptors (mAChR) located on target organs of the parasympathetic nervous system, nor on the nicotinic receptors at the skeletal neuromuscular junction, but acts as antagonist at the nicotinic acetylcholine receptors located in sympathetic and parasympathetic ganglia (nAChR).

Nicorandil is a vasodilator drug used to treat angina.
alpha-1 (α1) adrenergic receptors are G protein-coupled receptors (GPCRs) associated with the Gq heterotrimeric G protein. α1-adrenergic receptors are subdivided into three highly homologous subtypes, i.e., α1A-, α1B-, and α1D-adrenergic receptor subtypes. There is no α1C receptor. At one time, there was a subtype known as α1C, but it was found to be identical to the previously discovered α1A receptor subtype. To avoid confusion, naming was continued with the letter D. Catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) signal through the α1-adrenergic receptors in the central and peripheral nervous systems. The crystal structure of the α1B-adrenergic receptor subtype has been determined in complex with the inverse agonist (+)-cyclazosin.

Transient receptor potential cation channel, subfamily C, member 6 or Transient receptor potential canonical 6, also known as TRPC6, is a human gene encoding a protein of the same name. TRPC6 is a transient receptor potential channel of the classical TRPC subfamily.

The Prostacyclin receptor, also termed the prostaglandin I2 receptor or just IP, is a receptor belonging to the prostaglandin (PG) group of receptors. IP binds to and mediates the biological actions of prostacyclin (also termed Prostaglandin I2, PGI2, or when used as a drug, epoprostenol). IP is encoded in humans by the PTGIR gene. While possessing many functions as defined in animal model studies, the major clinical relevancy of IP is as a powerful vasodilator: stimulators of IP are used to treat severe and even life-threatening diseases involving pathological vasoconstriction.
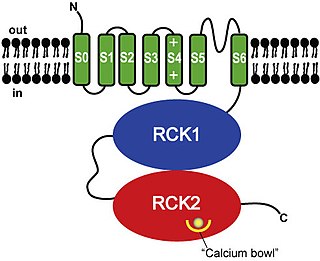
Calcium-activated potassium channel subunit alpha-1 also known as large conductance calcium-activated potassium channel, subfamily M, alpha member 1 (KCa1.1), or BK channel alpha subunit, is a voltage gated potassium channel encoded by the KCNMA1 gene and characterized by their large conductance of potassium ions (K+) through cell membranes.

Potassium voltage-gated channel, Shab-related subfamily, member 1, also known as KCNB1 or Kv2.1, is a protein that, in humans, is encoded by the KCNB1 gene.

ATP-sensitive inward rectifier potassium channel 12 is a lipid-gated ion channel that in humans is encoded by the KCNJ12 gene.

Potassium voltage-gated channel subfamily S member 3 (Kv9.3) is a protein that in humans is encoded by the KCNS3 gene. KCNS3 gene belongs to the S subfamily of the potassium channel family. It is highly expressed in pulmonary artery myocytes, placenta, and parvalbumin-containing GABA neurons in brain cortex. In humans, single-nucleotide polymorphisms of the KCNS3 gene are associated with airway hyperresponsiveness, whereas decreased KCNS3 mRNA expression is found in the prefrontal cortex of patients with schizophrenia.
A potassium channel opener is a type of drug which facilitates ion transmission through potassium channels.

Colin G. Nichols FRS is the Carl Cori Endowed Professor, and Director of the Center for Investigation of Membrane Excitability Diseases at Washington University in St. Louis, Missouri.















