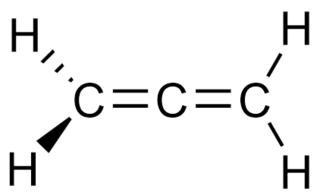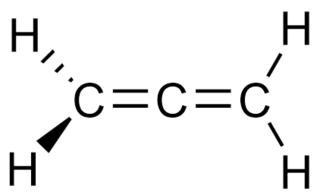
Allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres. Allenes are classified as cumulated dienes. The parent compound of this class is propadiene, which is itself also called allene. Compounds with an allene-type structure but with more than three carbon atoms are members of a larger class of compounds called cumulenes with X=C=Y bonding.

In chemistry, an aldehyde is an organic compound containing a functional group with the structure −C(H)=O. The functional group itself is known as an aldehyde or formyl group. Aldehydes are common and play important roles in the technology and biological spheres.
Ethanal (common name acetaldehyde) is an organic chemical compound with the formula CH3CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants. It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke. Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body.
Xanthine oxidase is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans.

Allicin is an organosulfur compound obtained from garlic, a species in the family Alliaceae. It was first isolated and studied in the laboratory by Chester J. Cavallito and John Hays Bailey in 1944. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic. The allicin generated is unstable and quickly changes into a series of other sulfur-containing compounds such as diallyl disulfide. Allicin is part of a defense mechanism against attacks by pests on the garlic plant.
A nitrile is any organic compound that has a −C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.
Propene, also known as propylene, is an unsaturated organic compound with the chemical formula . It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petroleum-like odor.

Enoyl-CoA-(∆) isomerase, also known as dodecenoyl-CoA-(∆) isomerase, 3,2-trans-enoyl-CoA isomerase, ∆3(cis),∆2(trans)-enoyl-CoA isomerase, or acetylene-allene isomerase, is an enzyme that catalyzes the conversion of cis- or trans-double bonds of coenzyme A (CoA) bound fatty acids at gamma-carbon to trans double bonds at beta-carbon as below:
Linoleic acid is an organic compound with the formula COOH(CH2)7CH=CHCH2CH=CH(CH2)4CH3. Both alkene groups are cis. It is a fatty acid sometimes denoted 18:2 (n-6) or 18:2 cis-9,12. A linoleate is a salt or ester of this acid.
In chemistry, homogeneous catalysis is catalysis in a solution by a soluble catalyst. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution. In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in distinct phases, typically solid-gas, respectively. The term is used almost exclusively to describe solutions and implies catalysis by organometallic compounds. Homogeneous catalysis is established technology that continues to evolve. An illustrative major application is the production of acetic acid. Enzymes are examples of homogeneous catalysts.
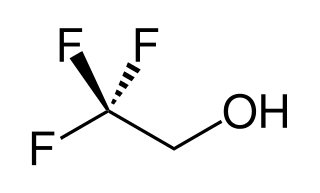
2,2,2-Trifluoroethanol is the organic compound with the formula CF3CH2OH. Also known as TFE or trifluoroethyl alcohol, this colourless, water-miscible liquid has a smell reminiscent of ethanol. Due to the electronegativity of the trifluoromethyl group, this alcohol exhibits a stronger acidic character compared to ethanol.
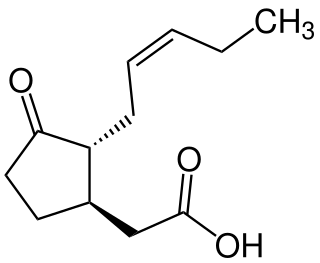
Jasmonic acid (JA) is an organic compound found in several plants including jasmine. The molecule is a member of the jasmonate class of plant hormones. It is biosynthesized from linolenic acid by the octadecanoid pathway. It was first isolated in 1957 as the methyl ester of jasmonic acid by the Swiss chemist Edouard Demole and his colleagues.

A sulfenic acid is an organosulfur compound and oxoacid with the general formula RSOH. It is the first member of the family of organosulfur oxoacids, which also include sulfinic acids and sulfonic acids, RSO2H and RSO3H, respectively. The base member of the sulfenic acid series with R = H is hydrogen thioperoxide.

Ribose 5-phosphate (R5P) is both a product and an intermediate of the pentose phosphate pathway. The last step of the oxidative reactions in the pentose phosphate pathway is the production of ribulose 5-phosphate. Depending on the body's state, ribulose 5-phosphate can reversibly isomerize to ribose 5-phosphate. Ribulose 5-phosphate can alternatively undergo a series of isomerizations as well as transaldolations and transketolations that result in the production of other pentose phosphates as well as fructose 6-phosphate and glyceraldehyde 3-phosphate.
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbonylation also refers to oxidation of protein side chains.

In enzymology, a 3-hydroxyacyl-CoA dehydrogenase (EC 1.1.1.35) is an enzyme that catalyzes the chemical reaction

In enzymology, an allene-oxide cyclase is an enzyme that belongs to the family of isomerases, specifically a class of other intramolecular oxidoreductases. The systematic name of this enzyme class is (9Z)-(13S)-12,13-epoxyoctadeca-9,11,15-trienoate isomerase (cyclizing).

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH (also written as CH3CO2H, C2H4O2, or HC2H3O2). Vinegar is no less than 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements.

Wine is a complex mixture of chemical compounds in a hydro-alcoholic solution with a pH around 4.

Methionine sulfoxide is the organic compound with the formula CH3S(O)CH2CH2CH(NH2)CO2H. It is an amino acid that occurs naturally although it is formed post-translationally.