
The endocrine system is a messenger system comprising feedback loops of the hormones released by internal glands of an organism directly into the circulatory system, regulating distant target organs. In vertebrates, the hypothalamus is the neural control center for all endocrine systems. In humans, the major endocrine glands are the thyroid gland, parathyroid gland, pituitary gland, pineal gland, the testes (male), ovaries (female), and the adrenal glands. The hypothalamus, pancreas, and thymus also function as endocrine glands, among other functions. Other organs, such as the kidneys, also have roles within the endocrine system by secreting certain hormones. The study of the endocrine system and its disorders is known as endocrinology.

The pancreatic islets or islets of Langerhans are the regions of the pancreas that contain its endocrine (hormone-producing) cells, discovered in 1869 by German pathological anatomist Paul Langerhans. The pancreatic islets constitute 1–2% of the pancreas volume and receive 10–15% of its blood flow. The pancreatic islets are arranged in density routes throughout the human pancreas, and are important in the metabolism of glucose.

Glucagon is a peptide hormone, produced by alpha cells of the pancreas. It raises the concentration of glucose and fatty acids in the bloodstream and is considered to be the main catabolic hormone of the body. It is also used as a medication to treat a number of health conditions. Its effect is opposite to that of insulin, which lowers extracellular glucose. It is produced from proglucagon, encoded by the GCG gene.
Delta cells are somatostatin-producing cells. They can be found in the stomach, intestine and the pancreatic islets. Delta cells comprise ca 5% of the cells in the islets but may interact with many more islet cells than suggested by their low numbers. In rodents, delta-cells are located in the periphery of the islets; in humans the islet architecture is generally less organized and delta-cells are frequently observed inside the islets as well. In both species, the peptide hormone Urocortin III (Ucn3) is a major local signal that is released from beta cells to induce the local secretion of somatostatin. It has also been suggested that somatostatin may be implicated in insulin-induced hypoglycaemia through a mechanism involving SGLT-2 receptors. Ghrelin can also strongly stimulate somatostatin secretion, thus indirectly inhibiting insulin release. Viewed under an electron microscope, delta-cells can be identified as cells with smaller and slightly more compact granules than beta cells.

Glucokinase is an enzyme that facilitates phosphorylation of glucose to glucose-6-phosphate. Glucokinase occurs in cells in the liver and pancreas of humans and most other vertebrates. In each of these organs it plays an important role in the regulation of carbohydrate metabolism by acting as a glucose sensor, triggering shifts in metabolism or cell function in response to rising or falling levels of glucose, such as occur after a meal or when fasting. Mutations of the gene for this enzyme can cause unusual forms of diabetes or hypoglycemia.
Alpha cells(α cells) are endocrine cells that are found in the Islets of Langerhans in the pancreas. Alpha cells secrete the peptide hormone glucagon in order to increase glucose levels in the blood stream.
Glucagonoma is a very rare tumor of the pancreatic alpha cells that results in the overproduction of the hormone, glucagon. Typically associated with a rash called necrolytic migratory erythema, weight loss, and mild diabetes mellitus, most people with glucagonoma contract it spontaneously. However, about 10% of cases are associated with multiple endocrine neoplasia type 1 (MEN-1) syndrome.

Multiple endocrine neoplasia type 1 (MEN-1) is one of a group of disorders, the multiple endocrine neoplasias, that affect the endocrine system through development of neoplastic lesions in pituitary, parathyroid gland and pancreas. Individuals suffering from this disorder are prone to developing multiple endocrine and nonendocrine tumors. It was first described by Paul Wermer in 1954.

Glucagon-like peptide-1 (GLP-1) is a 30- or 31-amino-acid-long peptide hormone deriving from the tissue-specific posttranslational processing of the proglucagon peptide. It is produced and secreted by intestinal enteroendocrine L-cells and certain neurons within the nucleus of the solitary tract in the brainstem upon food consumption. The initial product GLP-1 (1–37) is susceptible to amidation and proteolytic cleavage, which gives rise to the two truncated and equipotent biologically active forms, GLP-1 (7–36) amide and GLP-1 (7–37). Active GLP-1 protein secondary structure includes two α-helices from amino acid position 13–20 and 24–35 separated by a linker region.

The glucagon receptor is a 62 kDa protein that is activated by glucagon and is a member of the class B G-protein coupled family of receptors, coupled to G alpha i, Gs and to a lesser extent G alpha q. Stimulation of the receptor results in the activation of adenylate cyclase and phospholipase C and in increased levels of the secondary messengers intracellular cAMP and calcium. In humans, the glucagon receptor is encoded by the GCGR gene.

GNAS complex locus is a gene locus in humans. Its main product is the heterotrimeric G-protein alpha subunit Gs-α, a key component of G protein-coupled receptor-regulated adenylyl cyclase signal transduction pathways. GNAS stands for Guanine Nucleotide binding protein, Alpha Stimulating activity polypeptide.
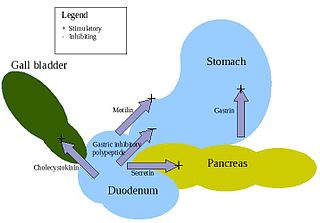
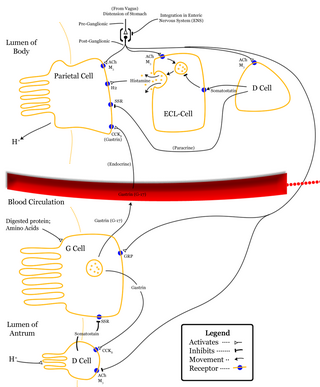
Enteroendocrine cells are specialized cells of the gastrointestinal tract and pancreas with endocrine function. They produce gastrointestinal hormones or peptides in response to various stimuli and release them into the bloodstream for systemic effect, diffuse them as local messengers, or transmit them to the enteric nervous system to activate nervous responses. Enteroendocrine cells of the intestine are the most numerous endocrine cells of the body. They constitute an enteric endocrine system as a subset of the endocrine system just as the enteric nervous system is a subset of the nervous system. In a sense they are known to act as chemoreceptors, initiating digestive actions and detecting harmful substances and initiating protective responses. Enteroendocrine cells are located in the stomach, in the intestine and in the pancreas. Microbiota plays key roles in the intestinal immune and metabolic responses in these enteroendocrine cells via their fermentation product, acetate.
Epsilon cells (ε-cells) are one of the five types of endocrine cells found in regions of the pancreas called Islets of Langerhans. Epsilon cells produce the hormone ghrelin that induces hunger. They were first discovered in mice. In humans, these cells compose less than 1% of all islet cells. They are connected by tight junctions that allow impermeability to water-soluble compounds.

The glucagon-like peptide-1 receptor (GLP1R) is a receptor protein found on beta cells of the pancreas and on neurons of the brain. It is involved in the control of blood sugar level by enhancing insulin secretion. In humans it is synthesised by the gene GLP1R, which is present on chromosome 6. It is a member of the glucagon receptor family of G protein-coupled receptors. GLP1R is composed of two domains, one extracellular (ECD) that binds the C-terminal helix of GLP-1, and one transmembrane (TMD) domain that binds the N-terminal region of GLP-1. In the TMD domain there is a fulcrum of polar residues that regulates the biased signaling of the receptor while the transmembrane helical boundaries and extracellular surface are a trigger for biased agonism.

Carboxypeptidase E (CPE), also known as carboxypeptidase H (CPH) and enkephalin convertase, is an enzyme that in humans is encoded by the CPE gene. This enzyme catalyzes the release of C-terminal arginine or lysine residues from polypeptides.

Somatostatin receptor type 2 is a protein that in humans is encoded by the SSTR2 gene.

Neurogenin-3 (NGN3) is a protein that in humans is encoded by the Neurog3 gene.
The fetal endocrine system is one of the first systems to develop during prenatal development of a human individual. The endocrine system arises from all three embryonic germ layers. The endocrine glands that produce the steroid hormones, such as the gonads and adrenal cortex, arise from the mesoderm. In contrast, endocrine glands that arise from the endoderm and ectoderm produce the amine, peptide, and protein hormones.

Pancreatic neuroendocrine tumours, often referred to as "islet cell tumours", or "pancreatic endocrine tumours" are neuroendocrine neoplasms that arise from cells of the endocrine (hormonal) and nervous system within the pancreas.

Mahvash disease is an autosomal recessive, hereditary pancreatic neuroendocrine tumor syndrome. The genetic defect that causes Mahvash disease is biallelic inactivating mutations of the glucagon receptor gene (GCGR). Mahvash disease was discovered by American physician Run Yu and his colleagues in 2008. Mahvash disease is very rare. There have been over 10 cases of Mahvash disease described by the end of 2018. Mahvash disease occurs in both females and males. Mahvash disease is also called “glucagon cell hyperplasia and neoplasia” or “glucagon cell adenomatosis” by some authors but Mahvash disease is a distinct disease entity while the two alternative terms are mostly histological descriptions. Some patients with “glucagon cell hyperplasia and neoplasia” do not have glucagon receptor mutations.















