
Aluminium is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, non-magnetic and ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of 26Al is used in radiodating.

Bauxite is a sedimentary rock with a relatively high aluminium content. It is the world's main source of aluminium and gallium. Bauxite consists mostly of the aluminium minerals gibbsite (Al(OH)3), boehmite (γ-AlO(OH)) and diaspore (α-AlO(OH)), mixed with the two iron oxides goethite (FeO(OH)) and haematite (Fe2O3), the aluminium clay mineral kaolinite (Al2Si2O5(OH)4) and small amounts of anatase (TiO2) and ilmenite (FeTiO3 or FeO.TiO2). Bauxite appears dull in luster and is reddish-brown, white, or tan in color.

An oxide is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. Metal oxides thus typically contain an anion of oxygen in the oxidation state of −2. Most of the Earth's crust consists of solid oxides, the result of elements being oxidized by the oxygen in air or in water. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 (called a passivation layer) that protects the foil from further corrosion. Certain elements can form multiple oxides, differing in the amounts of the element combining with the oxygen. Examples are carbon, iron, nitrogen (see nitrogen oxide), silicon, titanium, lithium, and aluminium. In such cases the oxides are distinguished by specifying the numbers of atoms involved, as in carbon monoxide and carbon dioxide, or by specifying the element's oxidation number, as in iron(II) oxide and iron(III) oxide.
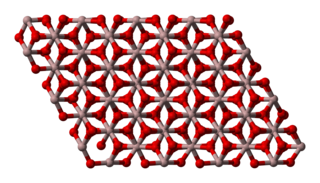
Aluminium oxide is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium(III) oxide. It is commonly called alumina and may also be called aloxide, aloxite, or alundum depending on particular forms or applications. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire. Al2O3 is significant in its use to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point.
In chemistry, an amphoteric compound is a molecule or ion that can react both as an acid and as a base. What exactly this can mean depends on which definitions of acids and bases are being used. The prefix of the word 'amphoteric' is derived from a Greek prefix amphi which means "both".
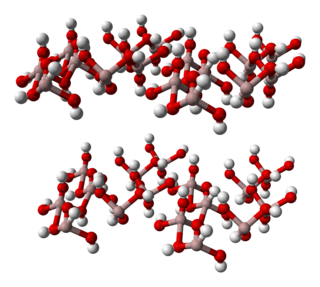
Aluminium hydroxide, Al(OH)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer polymorphs: bayerite, doyleite, and nordstrandite. Aluminium hydroxide is amphoteric, i.e., it has both basic and acidic properties. Closely related are aluminium oxide hydroxide, AlO(OH), and aluminium oxide or alumina (Al2O3), the latter of which is also amphoteric. These compounds together are the major components of the aluminium ore bauxite.
The Bayer process is the principal industrial means of refining bauxite to produce alumina (aluminium oxide) and was developed by Carl Josef Bayer. Bauxite, the most important ore of aluminium, contains only 30–60% aluminium oxide (Al2O3), the rest being a mixture of silica, various iron oxides, and titanium dioxide. The aluminium oxide must be purified before it can be refined to aluminium metal.
An acidic oxide is an oxide that either produces an acidic solution upon addition to water, or acts as an acceptor of hydroxide ions effectively functioning as a Lewis acid. Acidic oxides will typically have a low pKa and may be inorganic or organic. A commonly encountered acidic oxide, carbon dioxide produces an acidic solution when dissolved.

The capacitor plague was a problem related to a higher-than-expected failure rate of non-solid aluminium electrolytic capacitors, between 1999 and 2007, especially those from some Taiwanese manufacturers, due to faulty electrolyte composition that caused corrosion accompanied by gas generation, often rupturing the case of the capacitor from the build-up of pressure.

Aluminium chloride (AlCl3), also known as aluminium trichloride, describe compounds with the formula AlCl3(H2O)n (n = 0 or 6). They consist of aluminium and chlorine atoms in a 1:3 ratio, and one form also contains six waters of hydration. Both are white solids, but samples are often contaminated with iron(III) chloride, giving a yellow color.

Sodium aluminate is an inorganic chemical that is used as an effective source of aluminium hydroxide for many industrial and technical applications. Pure sodium aluminate (anhydrous) is a white crystalline solid having a formula variously given as NaAlO2, NaAl(OH)4 (hydrated), Na2O·Al2O3, or Na2Al2O4. Commercial sodium aluminate is available as a solution or a solid.
Other related compounds, sometimes called sodium aluminate, prepared by reaction of Na2O and Al2O3 are Na5AlO4 which contains discrete AlO45− anions, Na7Al3O8 and Na17Al5O16 which contain complex polymeric anions, and NaAl11O17, once mistakenly believed to be β-alumina, a phase of aluminium oxide.
In chemistry, an aluminate is a compound containing an oxyanion of aluminium, such as sodium aluminate. In the naming of inorganic compounds, it is a suffix that indicates a polyatomic anion with a central aluminum atom.

Chromium(III) oxide is an inorganic compound with the formula Cr
2O
3. It is one of the principal oxides of chromium and is used as a pigment. In nature, it occurs as the rare mineral eskolaite.
Aluminium silicate (or aluminum silicate) is a name commonly applied to chemical compounds which are derived from aluminium oxide, Al2O3 and silicon dioxide, SiO2 which may be anhydrous or hydrated, naturally occurring as minerals or synthetic. Their chemical formulae are often expressed as xAl2O3·ySiO2·zH2O. It is known as E number E559.
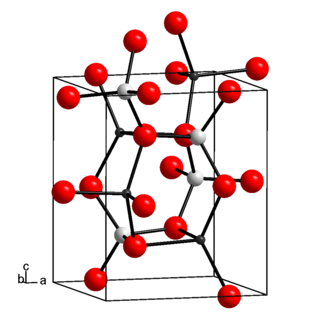
Lithium aluminate, also called lithium aluminium oxide, is an inorganic chemical compound, an aluminate of lithium. In microelectronics, lithium aluminate is considered as a lattice matching substrate for gallium nitride. In nuclear technology, lithium aluminate is of interest as a solid tritium breeder material, for preparing tritium fuel for nuclear fusion.
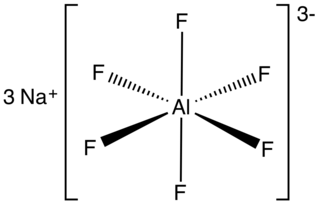
Sodium aluminium hexafluoride is an inorganic compound with formula Na3AlF6. This white solid, discovered in 1799 by Peder Christian Abildgaard (1740–1801), occurs naturally as the mineral cryolite and is used extensively in the industrial production of aluminium metal. The compound is the sodium (Na+) salt of the hexafluoroaluminate (AlF63−) ion.

The oxidation state of oxygen is −2 in almost all known compounds of oxygen. The oxidation state −1 is found in a few compounds such as peroxides. Compounds containing oxygen in other oxidation states are very uncommon: −1⁄2 (superoxides), −1⁄3 (ozonides), 0, +1⁄2 (dioxygenyl), +1, and +2.
Aluminium(I) oxide is a compound of aluminium and oxygen with the chemical formula Al2O. It can be prepared by heating the stable oxide Al2O3 with elemental silicon at 1800 °C under vacuum.
Aluminium carbonate (Al2(CO3)3), is a carbonate of aluminium. It is not well characterized; one authority says that simple carbonates of aluminium are not known. However related compounds are known, such as the basic sodium aluminium carbonate mineral dawsonite (NaAlCO3(OH)2) and hydrated basic aluminium carbonate minerals scarbroite (Al5(CO3)(OH)13•5(H2O)) and hydroscarbroite (Al14(CO3)3(OH)36•nH2O).

Aluminium (or aluminum) combines characteristics of pre- and post-transition metals. Since it has few available electrons for metallic bonding, like its heavier group 13 congeners, it has the characteristic physical properties of a post-transition metal, with longer-than-expected interatomic distances. Furthermore, as Al3+ is a small and highly charged cation, it is strongly polarizing and aluminium compounds tend towards covalency; this behaviour is similar to that of beryllium (Be2+), an example of a diagonal relationship. However, unlike all other post-transition metals, the underlying core under aluminium's valence shell is that of the preceding noble gas, whereas for gallium and indium it is that of the preceding noble gas plus a filled d-subshell, and for thallium and nihonium it is that of the preceding noble gas plus filled d- and f-subshells. Hence, aluminium does not suffer the effects of incomplete shielding of valence electrons by inner electrons from the nucleus that its heavier congeners do. Aluminium's electropositive behavior, high affinity for oxygen, and highly negative standard electrode potential are all more similar to those of scandium, yttrium, lanthanum, and actinium, which have ds2 configurations of three valence electrons outside a noble gas core: aluminium is the most electropositive metal in its group. Aluminium also bears minor similarities to the metalloid boron in the same group; AlX3 compounds are valence isoelectronic to BX3 compounds (they have the same valence electronic structure), and both behave as Lewis acids and readily form adducts. Additionally, one of the main motifs of boron chemistry is regular icosahedral structures, and aluminium forms an important part of many icosahedral quasicrystal alloys, including the Al–Zn–Mg class.












