Related Research Articles

In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula R−C(=O)−NR′R″, where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid with the hydroxyl group replaced by an amine group ; or, equivalently, an acyl (alkanoyl) group joined to an amine group.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.

The ammonium cation is a positively charged polyatomic ion with the chemical formula NH+4 or [NH4]+. It is formed by the protonation of ammonia. Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations, where one or more hydrogen atoms are replaced by organic or other groups.

In organic chemistry, acetyl is a functional group with the chemical formula −COCH3 and the structure −C(=O)−CH3. It is sometimes represented by the symbol Ac. In IUPAC nomenclature, acetyl is called ethanoyl, although this term is barely heard.
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the Nomenclature of Organic Chemistry. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.
In organic chemistry, a nitrile is any organic compound that has a −C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula −C4H9, derived from either of the two isomers (n-butane and isobutane) of butane.

Azo compounds are organic compounds bearing the functional group diazenyl.
Methylamine is an organic compound with a formula of CH3NH2. This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine.

Formamide is an amide derived from formic acid. It is a colorless liquid which is miscible with water and has an ammonia-like odor. It is chemical feedstock for the manufacture of sulfa drugs and other pharmaceuticals, herbicides and pesticides, and in the manufacture of hydrocyanic acid. It has been used as a softener for paper and fiber. It is a solvent for many ionic compounds. It has also been used as a solvent for resins and plasticizers. Some astrobiologists suggest that it may be an alternative to water as the main solvent in other forms of life.

In organic chemistry, an aminal or aminoacetal is a functional group or type of organic compound that has two amine groups attached to the same carbon atom: −C(NR2)(NR2)−.. A common aminal is bis(dimethylamino)methane, a colorless liquid that is prepared by the reaction of dimethylamine and formaldehyde:

Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2.
Isopentane, also called methylbutane or 2-methylbutane, is a branched-chain saturated hydrocarbon with five carbon atoms, with formula C
5H
12 or CH(CH
3)
2(C
2H
5).
Thiazolidine is a heterocyclic organic compound with the formula (CH2)3(NH)S. It is a 5-membered saturated ring with a thioether group and an amine group in the 1 and 3 positions. It is a sulfur analog of oxazolidine. Thiazolidine is a colorless liquid.
A chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry (IUPAC).
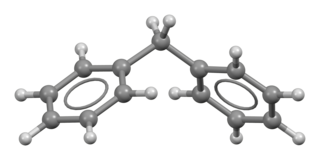
Diphenylmethane is an organic compound with the formula (C6H5)2CH2 (often abbreviated CH
2Ph
2). The compound consists of methane wherein two hydrogen atoms are replaced by two phenyl groups. It is a white solid.
In chemistry, an onium ion is a cation formally obtained by the protonation of mononuclear parent hydride of a pnictogen, chalcogen, or halogen. The oldest-known onium ion, and the namesake for the class, is ammonium, NH+4, the protonated derivative of ammonia, NH3.
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among the possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choosing between multiple possibilities in situations where it is important to decide on a unique name. It is intended for use in legal and regulatory situations.
Methylene is an organic compound with the chemical formula CH
2. It is a colourless gas that fluoresces in the mid-infrared range, and only persists in dilution, or as an adduct.

Cysteine methyl ester is the organic compound with the formula HSCH2CH(NH2)CO2CH3. A white solid, it is the methyl ester of the amino acid cysteine.
References
- ↑ Erwin Von Angerer, Norbert Knebel, Mario Kager, and Bernhard Ganss (1990): "1-(aminoalkyl)-2-phenylindoles as novel pure estrogen antagonists". Journal of Medicinal Chemistry, volume 33, issue 9, pages 2635–2640. doi : 10.1021/jm00171a045
- ↑ Ursula Bünzli-Trepp (2007): Systematic Nomenclature of Organic, Organometallic and Coordination Chemistry: Chemical-Abstracts Guidelines with IUPAC Recommendations and Many Trivial Names. EPFL Press, 636 pages. ISBN 9781420046151