Related Research Articles

Paracetamol (acetaminophen) is a non-opioid analgesic and antipyretic agent used to treat fever and mild to moderate pain. It is a widely used over the counter medication. Common brand names include Tylenol and Panadol.

Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) that is used to relieve pain, fever, and inflammation. This includes painful menstrual periods, migraines, and rheumatoid arthritis. It may also be used to close a patent ductus arteriosus in a premature baby. It can be used orally or intravenously. It typically begins working within an hour.

Over-the-counter (OTC) drugs are medicines sold directly to a consumer without a requirement for a prescription from a healthcare professional, as opposed to prescription drugs, which may be supplied only to consumers possessing a valid prescription. In many countries, OTC drugs are selected by a regulatory agency to ensure that they contain ingredients that are safe and effective when used without a physician's care. OTC drugs are usually regulated according to their active pharmaceutical ingredient (API) and strengths of final products.
Oxycodone/aspirin is a combination drug marketed by Endo Pharmaceuticals. It is a tablet containing a mixture of 325 mg of aspirin and 4.8355 mg of oxycodone HCl ; it is an opioid/non-opioid combination used to treat moderate to moderately severe pain. The safety of the combination during pregnancy has not been established, although aspirin is generally contraindicated during pregnancy, and the drug has been placed in pregnancy category D. Inactive ingredients include D&C Yellow 10, FD&C Yellow 6, microcrystalline cellulose, and corn starch. Percodan was first marketed by DuPont Pharmaceuticals and prescribed in the United States in 1950. Once a widely prescribed painkiller, it has largely been replaced by alternative oxycodone compounds containing paracetamol (acetaminophen) instead of aspirin, such as Percocet.

Dihydrocodeine is a semi-synthetic opioid analgesic prescribed for pain or severe dyspnea, or as an antitussive, either alone or compounded with paracetamol (acetaminophen) or aspirin. It was developed in Germany in 1908 and first marketed in 1911.

Excedrin is an over-the-counter headache pain reliever, typically in the form of tablets or caplets. It contains paracetamol, aspirin and caffeine. It was manufactured by Bristol-Myers Squibb until it was purchased by Novartis in July 2005 along with other products from BMS's over-the-counter business. As of March 2015, GSK holds majority ownership of Excedrin through a joint venture transaction with Novartis. On 18 July 2022, GSK spun off its consumer healthcare business to Haleon.
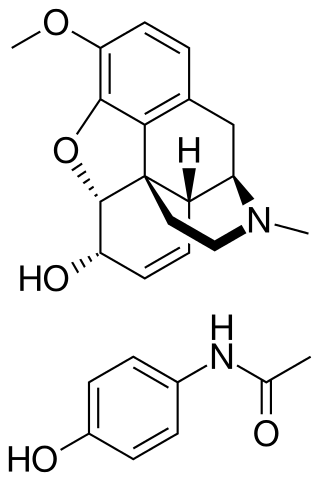
Codeine/paracetamol, also called codeine/acetaminophen and co-codamol, is a compound analgesic, comprising codeine phosphate and paracetamol (acetaminophen). Codeine/paracetamol is used for the relief of mild to moderate pain when paracetamol or non-steroidal anti-inflammatory drugs alone do not sufficiently relieve symptoms.
Goody's Powder, also called Goody's Headache Powders, is an over-the-counter aspirin/paracetamol/caffeine–based pain reliever, in single-dose powder form, which is marketed and sold by Prestige Brands. The powder delivery saves the time needed for the patient's digestive system to break down a tablet or capsule, ostensibly causing the medication to work faster. Goody's Extra Strength Powder consists of aspirin, caffeine, and paracetamol (acetaminophen) in a formula identical to that of Excedrin, a product of Novartis, but in the no-digestion-needed powder form.

Anacin is an American brand of analgesic that is manufactured by Prestige Consumer Healthcare. Its product formerly contained acetanilide, which resulted in a Not Acceptable rating from Consumer Reports. At present it contains aspirin and caffeine.
Propyphenazone/paracetamol/caffeine is an analgesic combination indicated for the management of headache. It contains the analgesics propyphenazone and paracetamol and the stimulant caffeine.
Compound analgesics are those with multiple active ingredients; they include many of the stronger prescription analgesics.

Solpadeine is the brand name of a range of analgesic medication containing various amounts of paracetamol, ibuprofen, caffeine and codeine, made by Omega Pharma. The range was previously made by GlaxoSmithKline, which sold its portfolio of over-the-counter drugs to Omega Pharma in 2012.

Codeine is an opiate and prodrug of morphine mainly used to treat pain, coughing, and diarrhea. It is also commonly used as a recreational drug. It is found naturally in the sap of the opium poppy, Papaver somniferum. It is typically used to treat mild to moderate degrees of pain. Greater benefit may occur when combined with paracetamol (acetaminophen) or a nonsteroidal anti-inflammatory drug (NSAID) such as aspirin or ibuprofen. Evidence does not support its use for acute cough suppression in children. In Europe, it is not recommended as a cough medicine in those under 12 years of age. It is generally taken by mouth. It typically starts working after half an hour, with maximum effect at two hours. Its effects last for about four to six hours. Codeine exhibits abuse potential similar to other opioid medications, including a risk of habituation and overdose.
Aspirin, an organic compound that does not occur in nature, was first synthesised in 1899.
Paracetamol/metoclopramide hydrochloride is an oral fixed dose combination prescription medication containing the analgesic paracetamol (500 mg) and the anti-emetic metoclopramide hydrochloride (5 mg). Formulated as a tablet and as sachets of a water-soluble powder, it is sold under the trade name Paramax by Sanofi-Synthelabo, and in Switzerland as Migraeflux MCP, in Australia it is sold as Meteclomax and Anagraine.

Hydrocodone/ibuprofen (INNs), sold under the brand name Vicoprofen, is a fixed-dose combination analgesic medication used in short-term therapy to relieve severe pain. Vicoprofen combines the analgesic and antitussive properties of hydrocodone with the analgesic, anti-inflammatory, and antipyretic properties of ibuprofen. In contrast to hydrocodone/acetaminophen combination analgesics such as Vicodin, this hydrocodone/ibuprofen avoids some of the liver toxicity which may occur from acetaminophen, but still presents significant dangers in hydrocodone overdose, namely respiratory depression. Vicoprofen is supplied in a fixed dose combination tablet which contains hydrocodone bitartrate, USP 7.5 mg with ibuprofen, USP 200 mg. Additional strengths of generic Vicoprofen are now available, in combinations of 5 mg/200 mg and 10 mg/200 mg respectively.
Ibuprofen/paracetamol, sold under the brand name Combogesic among others, is a fixed-dose combination of two medications, ibuprofen, a non-steroidal anti-inflammatory drug (NSAID); and paracetamol (acetaminophen), an analgesic and antipyretic. It is available as a generic medication.
Aspirin/paracetamol/caffeine is a combination drug for the treatment of pain, especially tension headache and migraine. It contains aspirin, a non-steroidal anti-inflammatory drug; paracetamol (acetaminophen), an analgesic; and caffeine, a stimulant.
Nurofen is a brand name range of pain-relief medication containing ibuprofen made by the British multinational Reckitt. Introduced in 1983, the Nurofen brand was acquired following Reckitt Benckiser's acquisition of Boots healthcare international in 2005 for £1.93 billion, which included Nurofen, Strepsils, and Clearasil. The brand is primarily marketed and sold in the United Kingdom, other parts of Europe, South Africa, Australia and New Zealand. In 2016 it was the biggest selling branded over-the-counter medication sold in Great Britain, with sales of £116.8 million.
References
- ↑ "Home". anadin.co.uk.
- ↑ http://www.brandrepublic.com/InDepth/Features/188772/Superbrands-case-studies-Anadin/ Archived 2009-08-18 at the Wayback Machine Superbrands case studies: Anadin
- ↑ "The great medicine rip-off". The Independent. 15 April 2008.
- ↑ "Anadin brand". brandrepublic.com. Retrieved April 9, 2016.