Related Research Articles

The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae, and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa.

Susan Lee Lindquist, ForMemRS was an American professor of biology at MIT specializing in molecular biology, particularly the protein folding problem within a family of molecules known as heat-shock proteins, and prions. Lindquist was a member and former director of the Whitehead Institute and was awarded the National Medal of Science in 2010.
Heat shock proteins (HSP) are a family of proteins produced by cells in response to exposure to stressful conditions. They were first described in relation to heat shock, but are now known to also be expressed during other stresses including exposure to cold, UV light and during wound healing or tissue remodeling. Many members of this group perform chaperone functions by stabilizing new proteins to ensure correct folding or by helping to refold proteins that were damaged by the cell stress. This increase in expression is transcriptionally regulated. The dramatic upregulation of the heat shock proteins is a key part of the heat shock response and is induced primarily by heat shock factor (HSF). HSPs are found in virtually all living organisms, from bacteria to humans.
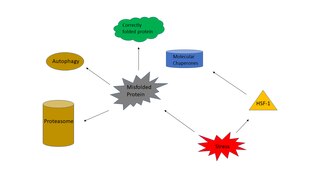
The heat shock response (HSR) is a cell stress response that increases the number of molecular chaperones to combat the negative effects on proteins caused by stressors such as increased temperatures, oxidative stress, and heavy metals. In a normal cell, proteostasis must be maintained because proteins are the main functional units of the cell. Many proteins take on a defined configuration in a process known as protein folding in order to perform their biological functions. If these structures are altered, critical processes could be affected, leading to cell damage or death. The heat shock response can be employed under stress to induce the expression of heat shock proteins (HSP), many of which are molecular chaperones, that help prevent or reverse protein misfolding and provide an environment for proper folding.

In cellular biology, stress granules are dense aggregations in the cytosol composed of proteins and RNAs that appear when the cell is under stress. The RNA molecules stored are stalled translation pre-initiation complexes: failed attempts to make protein from mRNA. Stress granules are 100–200 nm in size, not surrounded by membrane, and associated with the endoplasmatic reticulum. Note that there are also nuclear stress granules. This article is about the cytosolic variety.

Peter Walter is a German-American molecular biologist and biochemist and is Director of the Bay Area Institute of Science at Altos Labs, Professor at the University of California, San Francisco (UCSF). He was a Howard Hughes Medical Institute (HHMI) Investigator until 2022.

Heat shock factor 1 (HSF1) is a protein that in humans is encoded by the HSF1 gene. HSF1 is highly conserved in eukaryotes and is the primary mediator of transcriptional responses to proteotoxic stress with important roles in non-stress regulation such as development and metabolism.

X-box binding protein 1, also known as XBP1, is a protein which in humans is encoded by the XBP1 gene. The XBP1 gene is located on chromosome 22 while a closely related pseudogene has been identified and localized to chromosome 5. The XBP1 protein is a transcription factor that regulates the expression of genes important to the proper functioning of the immune system and in the cellular stress response.

Activating transcription factor 6, also known as ATF6, is a protein that, in humans, is encoded by the ATF6 gene and is involved in the unfolded protein response.

DNA damage-inducible transcript 3, also known as C/EBP homologous protein (CHOP), is a pro-apoptotic transcription factor that is encoded by the DDIT3 gene. It is a member of the CCAAT/enhancer-binding protein (C/EBP) family of DNA-binding transcription factors. The protein functions as a dominant-negative inhibitor by forming heterodimers with other C/EBP members, preventing their DNA binding activity. The protein is implicated in adipogenesis and erythropoiesis and has an important role in the cell's stress response.

Nuclear factor of activated T-cells 5, also known as NFAT5 and sometimes TonEBP, is a human gene that encodes a transcription factor that regulates the expression of genes involved in the osmotic stress.

Krueppel-like factor 9 is a protein that in humans is encoded by the KLF9 gene. Previously known as Basic Transcription Element Binding Protein 1, Klf9 is part of the Sp1 C2H2-type zinc finger family of transcription factors. Several previous studies showed Klf9-related regulation of animal development, including cell differentiation of B cells, keratinocytes, and neurons. Klf9 is also a key transcriptional regulator for uterine endometrial cell proliferation, adhesion, and differentiation, all factors that are essential during the process of pregnancy and are turned off during tumorigenesis.
Richard I. Morimoto is a Japanese American molecular biologist. He is the Bill and Gayle Cook Professor of Biology and Director of the Rice Institute for Biomedical Research at Northwestern University.
Zhijian "James" Chen is a Chinese-American biochemist and Professor in the Department of Molecular Biology at University of Texas Southwestern Medical Center. He is best known for his discovery of mechanisms by which nucleic acids trigger innate and autoimmune responses from the interior of a cell, work for which he received the 2019 Breakthrough Prize in Life Sciences.
Cellular stress response is the wide range of molecular changes that cells undergo in response to environmental stressors, including extremes of temperature, exposure to toxins, and mechanical damage. Cellular stress responses can also be caused by some viral infections. The various processes involved in cellular stress responses serve the adaptive purpose of protecting a cell against unfavorable environmental conditions, both through short term mechanisms that minimize acute damage to the cell's overall integrity, and through longer term mechanisms which provide the cell a measure of resiliency against similar adverse conditions.

Gisela Storz is a microbiologist at the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) at the National Institutes of Health (NIH). She is a member of the National Academy of Sciences.
Patrick D'Silva is an Indian cell biologist, biochemist, and an associate professor at the Molecular Chaperone Lab of the Indian Institute of Science. He is known for his medical discoveries related to neurodegenerative diseases and cancer biology. The Department of Biotechnology of the Government of India awarded him the National Bioscience Award for Career Development, one of the highest Indian science awards, for his contributions to biosciences, in 2014.
Ann M. Nardulli was an American endocrinologist known for her research into the role of estrogen in breast cancer.
Jian-Kang Zhu is a plant scientist, researcher and academic. He is a Senior Principal Investigator in the Shanghai Center for Plant Stress Biology, Chinese Academy of Sciences (CAS). He is also the Academic Director of CAS Center of Excellence in Plant Sciences.
Teresa Shu-Fong Wang is an American biochemist.